

ACR Manual on Contrast
Media
2024
ACR Committee on Drugs
and Contrast Media
© Copyright 2024 American College of Radiology

TABLE OF CONTENTS
Topic
Page
1.
Preface
1
2.
Version History
2
3.
Introduction
4
4.
Patient Selection and Preparation Strategies Before Contrast Medium Administration
5
5.
Fasting Prior to Intravascular Contrast Media Administration
14
6.
Safe Injection of Contrast Media
15
7.
Extravasation of Contrast Media – 2022 Evidence Based Update
19
8.
Allergic-Like And Physiologic Reactions to Intravascular Iodinated Contrast Media
29
9.
Contrast Media Warming
36
10.
Post-Contrast Acute Kidney Injury and Contrast-Induced Nephropathy in Adults
40
11.
Metformin
51
12.
Contrast Media in Children
54
•
Ferumoxytol as MRI Contrast Medium (New 2024 addition)
64
13.
Gastrointestinal (GI) Contrast Media in Adults: Indications and Guidelines – 2024 Update
69
14.
ACR–ASNR Position Statement on the Use of Gadolinium Contrast Agents
79
15.
Adverse Reactions to Gadolinium-Based Contrast Media
80
•
Gadolinium Pregnancy Screening Statement (2023 addition)
83
16.
Nephrogenic Systemic Fibrosis (NSF)
84
•
ACR Manual Classification of Gadolinium-Based Agents Relative to Nephrogenic Systemic Fibrosis
89
17.
Ultrasound Contrast Media
92
18.
Treatment of Contrast Reactions
95
19.
Administration of Contrast Media to Pregnant or Potentially Pregnant Patients
97
20.
Administration of Contrast Media to Women Who are Breast-Feeding – 2024 Evidenced Based Update
101
Table 1 – Categories of Acute Reactions
104
Table 2 – Treatment Of Acute Reactions to Contrast Media In Children
106
Table 3 – Management Of Acute Reactions to Contrast Media In Adults
113
Table 4 – Equipment For Contrast Reaction Kits in Radiology
121
Appendix A – Contrast Media Specifications
123
ACR MANUAL ON CONTRAST MEDIA – PREFACE 1
PREFACE
This edition of the ACR Manual on Contrast Media replaces all earlier editions. It is being published as a web-based document
only so it can be updated as frequently as needed.
This manual was developed by the ACR Committee on Drugs and Contrast Media of the ACR Commission on Quality and
Safety as a guide for radiologists to enhance the safe and effective use of contrast media. The Committee offers this document
to practicing radiologists as a consensus of scientific evidence and clinical experience concerning the use of contrast media.
Suggestions for patient screening, premedication, recognition of adverse reactions, and emergency treatment of such reactions
are emphasized. Its major purpose is to provide useful information regarding contrast media used in daily practice.
The editorial staff sincerely thanks all who have contributed their knowledge and valuable time to this publication.
Members of the ACR Committee on Drugs and Contrast Media are:
Carolyn Wang, MD, Chair
Robert J. McDonald, MD
Daniella Asch, MD
Jennifer McDonald, PhD
Mustafa Shadi Rifaat Bashir, MD
Benjamin Mervak, MD
Michael James Callahan, MD
Jeffrey Newhouse, MD, FACR
Jonathan Dillman, MD, MSc
Jay Pahade, MD
James Ellis, MD, FACR
Jennifer G. Schopp, MD
Monica Forbes-Amrhein, MD
Prasad R. Shankar, MD
Leah Gilligan, MD
Kerry L. Thomas, MD
Pranay Krishnan, MD
Finally, the committee wishes to recognize the efforts of supporting members of the ACR staff.
The manual is copyright protected and the property of the American College of Radiology. Any reproduction or attempt to
sell this manual is strictly prohibited absent the express permission of the American College of Radiology.
ACR MANUAL ON CONTRAST MEDIA – VERSION HISTORY 2
VERSION HISTORY
2024
Version 2024 of the ACR Manual on Contrast Media was published in July 2024 as a web-based product. Content changes may
take place as a result of changes in technology, clinical treatment, or other evidence-based decisions from the contrast committee.
The following changes have been made:
Last Updated
Chapter
Change
2010
Introduction
Updated
2013
Chapter 7 – Allergic-like and Physiologic Reactions to Intravascular Iodinated Contrast
Media
Updated
2013
Chapter 8 – Contrast Media in Children
Updated
2013
Chapter 12 – Gastrointestinal (GI) Contrast Media in Adults: indications and
Guidelines
Updated
2013
Chapter 19 - Administration of Contrast Media to Women Who Are Breast-Feeding
Updated
2014
Chapter 11- Contrast Media in Children
Updated
2014
Appendix A
Updated
2015
Preface
Updated
2016
Chapter 13– ACR-ASNR Position Statement on the Use of Gadolinium Contrast
Agents
A collaborative statement on
gadolinium deposition was added to the
manual
2016
Table 1 – Indications for Use of Iodinated Contrast Media
Deleted
2016
Table 2 – Organ and System-Specific Adverse Effects from the Administration of
Iodine-Based or Gadolinium-Based Contrast Agents
Deleted
2016
Chapter 9 – Metformin
Updated footnote based on new FDA advisory
2016
Chapter 14 – Injection of Contrast Media
New section on intra-osseous injection
2016
Chapter 13 – ACR-ASNR Position Statement on the Use of Gadolinium Contrast
Agents
New Chapter added
2017
Chapter 15 – Nephrogenic Systemic Fibrosis
Updated
2017
Chapter 4 – Patient Selection and Preparation Strategies
Updated
2017
Chapter 17 – Ultrasound Contrast Media
New chapter added
2017
Chapter 19 – Administration of Contrast Media to Pregnant or Potentially Pregnant
Patients
Updated
2018
Chapter 5 – Injection of Contrast Media
Updated
2018
Chapter 6 – Extravasation of Contrast Media
Updated
2020
Chapter 18 – Treatment of Contrast Reactions
Updated
2020
Table 4 – Equipment for Contrast Reaction Kits in Radiology
Updated
ACR MANUAL ON CONTRAST MEDIA – VERSION HISTORY 3
2020
Appendix (Approved Contrast Media Agents)
Updated
2021
Chapter 5 - Fasting Prior to Intravascular Contrast Media Administration
New added chapter
2021
Chapter 10 - Post-Contrast Acute Kidney Injury and Contrast-Induced
Nephropathy in Adults
ACR-NKF Consensus language harmonization
update with chapter title change
2021
Chapter 16 - Nephrogenic Systemic Fibrosis (NSF)
ACR-NKF Consensus language harmonization
update
2022
Chapter 7 – Extravasation of Contrast Media
Evidence based update with recommendations
and strength of evidence
2022
Chapter 15 - Adverse Reactions To Gadolinium-Based Contrast Media
New Gadolinium Pregnancy Screening
Statement
2023
Chapter 16 - Nephrogenic Systemic Fibrosis (NSF)
Updated Calculating eGFR for Adults
2023
TABLE 1. ACR Manual Classification of Gadolinium-Based Agents Relative to
Nephrogenic Systemic Fibrosis
Gadopiclenol Update
2023
Appendix A – Contrast Media Specifications
Gadopiclenol Update
2024
TABLE 1. ACR Manual Classification of Gadolinium-Based Agents Relative to
Nephrogenic Systemic Fibrosis
Eovist Update
2024
Chapter 12 – Contrast Media in Children
Update and New Ferumoxytol addition
2024
Chapter 13 - Gastrointestinal (GI) Contrast Media in Adults: indications and
Guidelines
Updated
2024
Chapter 20 - Administration of Contrast Media to Women Who are Breast-Feeding
Evidence based update with recommendations
and strength of evidence
ACR MANUAL ON CONTRAST MEDIA – INTRODUCTION 4
INTRODUCTION
Various forms of contrast media have been used to improve medical imaging. Their value has long been recognized, as
attested to by their common daily use in imaging departments worldwide. Like all other pharmaceuticals, however, these
agents are not completely devoid of risk. The major purpose of this manual is to assist radiologists in recognizing and
managing the small but real risks inherent in the use of contrast media.
Adverse side effects from the administration of contrast media vary from minor physiological disturbances to rare severe
life-threatening situations. Preparation for prompt treatment of contrast media reactions must include preparation for the
entire spectrum of potential adverse events and include prearranged response planning with availability of appropriately
trained personnel, equipment, and medications. Therefore, such preparation is best accomplished prior to approving and
performing these examinations. Additionally, an ongoing quality assurance and quality improvement program for all
radiologists and technologists and the requisite equipment are recommended. Thorough familiarity with the presentation
and emergency treatment of contrast media reactions must be part of the environment in which all intravascular contrast
media are administered.
Millions of radiological examinations assisted by intravascular contrast media are conducted each year in North America.
Although adverse side effects are infrequent, a detailed knowledge of the variety of side effects, their likelihood in
relationship to pre-existing conditions, and their treatment is required to insure optimal patient care.
As would be appropriate with any diagnostic procedure, preliminary considerations for the referring physician and the
radiologist include:
1.
Assessment of patient risk versus potential benefit of the contrast-assisted examination.
2.
Imaging alternatives that would provide the same or better diagnostic information.
3.
Assurance of a valid clinical indication for each contrast medium administration.
Because of the documented low incidence of adverse events, intravenous injection of contrast media may be exempted from
the need for informed consent, but this decision should be based on state law, institutional policy, and departmental policy.
Usage Note: In this manual, the term “low-osmolality” in reference to radiographic iodinated contrast media is intended to
encompass both low-osmolality and iso-osmolality media, the former having osmolality approximately twice that of human
serum, and the latter having osmolality approximately that of human serum at conventionally used iodine concentrations for
vascular injection. Also, unless otherwise obvious in context, this manual focuses on issues concerning radiographic
iodinated contrast media.

PATIENT SELECTION AND PREPARATION STRATEGIES BEFORE CONTRAST MEDIUM ADMINISTRATION 5
PATIENT SELECTION AND PREPARATION STRATEGIES BEFORE
CONTRAST MEDIUM ADMINISTRATION
General Considerations
The approach to patients about to undergo a contrast-enhanced examination has four general goals:
1)
Ensure that the administration of contrast is appropriate for the patient and the indication
2)
Balance the likelihood of an adverse event with the benefit of the examination
3)
Promote efficient and accurate diagnosis and treatment
4)
Be prepared to treat a reaction should one occur (see Tables 2, and 3)
Achieving these aims depends on obtaining an appropriate and adequate history for each patient, considering the risks and
benefit of using or avoiding contrast medium, preparing the patient appropriately for the examination, having equipment
available to treat reactions, and ensuring that personnel with sufficient expertise are available to treat severe reactions.
The history obtained should focus on identification of factors that may indicate either a contraindication to contrast media use
or an increased likelihood of an adverse event. Screening questions should include historical elements that will affect decision-
making in the patient selection and preparation period.
Risk Factors for Adverse Reactions to Intravenous
Contrast Media Primary Considerations
Allergic-like reactions to modern iodinated and gadolinium-based contrast medium are uncommon (iodinated: 0.6% aggregate
[1], 0.04% severe [2]; gadolinium-based: 0.01-0.22% aggregate [2], 0.008% severe) [3]. Risk factors exist that increase the risk
of a contrast reaction. These generally increase the likelihood of a reaction by less than one order of magnitude, effectively
increasing the risk that an uncommon event will occur, but not guaranteeing a reaction will take place. The following are some
examples:
Allergy: Patients who have had a prior allergic-like reaction or unknown-type reaction (i.e., a reaction of unknown
manifestation) to contrast medium have an approximately 5-fold increased risk of developing a future allergic-like reaction if
exposed to the same class of contrast medium again [2]. A prior allergic-like or unknown type reaction to the same class of
contrast medium is considered the greatest risk factor for predicting future adverse events.
In general, patients with unrelated allergies are at a 2- to 3-fold increased risk of an allergic-like contrast reaction, but due to
the modest increased risk, restricting contrast medium use or premedicating solely on the basis of unrelated allergies is not
recommended. Patients with shellfish or povidone-iodine (e.g., Betadine
®
) allergies are at no greater risk from iodinated contrast
medium than are patients with other allergies (i.e., neither is a significant risk factor) [4,5].
There is no cross-reactivity between different classes of contrast medium. For example, a prior reaction to gadolinium-based
contrast medium does not predict a future reaction to iodinated contrast medium, or vice versa, more than any other unrelated
allergy.
Asthma: A history of asthma increases the likelihood of an allergic-like contrast reaction [2,6].
Patients with asthma may be more prone to develop bronchospasm. Due to the modest increased risk, restricting contrast
medium use or premedicating solely on the basis of a history of asthma is not recommended.
Renal Insufficiency: Screening and selection strategies to mitigate the possible risks of the non-allergic adverse events of
contrast-induced nephrotoxicity (CIN) and nephrogenic systemic fibrosis (NSF) can be found in the Chapters on Post-Contrast
Acute Kidney Injury and Contrast Induced Nephropathy in Adults and Nephrogenic Systemic Fibrosis.
Cardiac Status: Patients with severe cardiac disease may be at increased risk of a non-allergic cardiac event if an allergic-like
or non-allergic contrast reaction occurs. These include symptomatic patients (e.g., patients with angina or congestive heart
PATIENT SELECTION AND PREPARATION STRATEGIES BEFORE CONTRAST MEDIUM ADMINISTRATION 6
failure symptoms with minimal exertion) and also patients with severe aortic stenosis, cardiac arrhythmias, primary pulmonary
hypertension, or severe but compensated cardiomyopathy. Due to the modest increased risk, restricting contrast medium use
or premedicating solely on the basis of a patient’s cardiac status is not recommended.
Anxiety: There is some evidence that contrast reactions are more common in anxious patients [7]. Reassuring an anxious
patient before contrast medium injection may mitigate the likelihood of a mild contrast reaction.
Other Historical and Pre-Procedure Considerations
Age and Gender: Infants, neonates, children, and the elderly have lower reaction rates than middle-aged patients [1,8]. Male
patients have lower reaction rates than female patients. Due to the modest increased risk, restricting contrast medium use or
premedicating solely on the basis of patient age or gender is not recommended.
Beta-Blockers: Some have suggested that use of beta-blockers lowers the threshold for contrast reactions, increases the
severity of contrast reactions, and reduces the responsiveness of treatment with epinephrine [9]. Due to the modest increased
risk, restricting contrast medium use or premedicating solely on the basis of beta-blocker use is not recommended. Patients
on beta-blocker therapy do not need to discontinue their medication(s) prior to contrast medium administration.
Sickle-Cell Trait/Disease: Some have suggested that contrast medium exposure to patients with sickle cell trait or sickle
cell disease might increase the risk of an acute sickle crisis; however, there is no evidence this occurs with modern
iodinated or gadolinium-based contrast medium [10]. Therefore, restricting contrast medium use or premedicating solely
on the basis of sickle cell trait or sickle cell disease is not recommended.
Pheochromocytoma: There is no evidence that IV administration of modern iodinated or gadolinium-based contrast medium
increases the risk of hypertensive crisis in patients with pheochromocytoma [11]. Therefore, restricting contrast medium use
or premedicating solely on the basis of a history of pheochromocytoma is not recommended. Direct injection of any type of
contrast medium into the adrenal or renal arteries in a patient with pheochromocytoma has not been adequately studied and is
of unknown risk.
Myasthenia Gravis: There is a questionable relationship between IV iodinated contrast medium and exacerbations of
myasthenic symptoms in patients with myasthenia gravis. While one retrospective study showed no immediate increase in
myasthenic symptoms following the administration of iodinated or gadolinium-based contrast medium [12], another that
searched for myasthenic exacerbations occurring up to 45 days after a CT scan found that IV non-ionic iodinated contrast
medium was associated with an acute (within 1 day of contrast administration) myasthenic exacerbation in approximately 6%
of patients (compared to a 1% acute exacerbation rate in patients who had undergone non-contrast CT, p=0.01) [13]. However,
that study was retrospective, and the number of events was small. Premedication is not recommended solely on the basis of a
history of myasthenia gravis. It is controversial whether iodinated contrast medium should be considered a relative
contraindication in patients with myasthenia gravis.
Hyperthyroidism: Patients with a history of hyperthyroidism can develop thyrotoxicosis after exposure to iodinated contrast
medium, but this complication is rare [14]. Therefore, restricting contrast medium use or premedicating solely on the basis of
a history of hyperthyroidism is not recommended. However, two special situations may affect this:
1.
In patients with acute thyroid storm, iodinated contrast medium exposure can potentiate thyrotoxicosis; in such
patients, iodinated contrast medium should be avoided. Corticosteroid premedication in this setting is unlikely to
be helpful.
2.
In patients considering radioactive iodine therapy or in patients undergoing radioactive iodine imaging of the
thyroid gland, administration of iodinated contrast medium can interfere with uptake of the treatment and diagnostic
dose. If iodinated contrast medium was administered, a washout period is suggested to minimize this interaction.
The washout period is ideally 3-4 weeks for patients with hyperthyroidism, and 6 weeks for patients with
hypothyroidism [15,16].
Normal Thyroid Function: Iodinated contrast medium does not affect thyroid function test results in patients with a
normally functioning thyroid gland [14]. Multiple studies have shown that a single dose of iodinated contrast medium
administered to a pregnant mother has no effect on neonatal thyroid function.
PATIENT SELECTION AND PREPARATION STRATEGIES BEFORE CONTRAST MEDIUM ADMINISTRATION 7
Angiography: Iso-osmolality contrast media (IOCM) are associated with the least amount of vasospasm and the least
peripheral discomfort for peripheral angiograms [17]. Concomitant use of iodinated contrast medium with certain intra-
arterial medications (e.g., papaverine) may lead to precipitation of contrast medium and crystal or thrombus formation.
Decisions about the use and timing of such medication are outside the scope of this document.
Pretesting
Intradermal skin testing with contrast media to predict the likelihood of adverse reactions has not been shown to be useful
in minimizing reaction risk [18-20].
Corticosteroid Premedication
The purpose of corticosteroid premedication is to mitigate the likelihood of an allergic-like reaction in high- risk patients.
Etiology of Hypersensitivity Contrast Reactions: The etiological mechanism of most immediate hypersensitivity contrast
reactions is incompletely understood [21]. It is known, however, that approximately 90% of such adverse reactions are
associated with direct release of histamine and other mediators from circulating basophils and eosinophils. It is also generally
accepted that most adverse allergic-like reactions are not associated with the presence of increased IgE, and therefore are
unlikely to be typical IgE-mediated hypersensitivity reactions. However, some studies show evidence of IgE mediation [18].
No antibodies to IV contrast media have been consistently identified, and according to skin testing and basophil activation,
IgE-mediated allergy is uncommon, for example occurring in 4% of patients having anaphylaxis symptoms [19]. This likely
explains why patients who have never been exposed to contrast media can experience a severe hypersensitivity reaction on
first exposure. Prior sensitization is not required for a contrast reaction to occur.
Pathophysiologic explanations for allergic-like hypersensitivity reactions include activation of mast cells and basophils
releasing histamine, activation of the contact and complement systems, conversion of L-arginine into nitric oxide, activation
of the XII clotting system leading to production of bradykinin [10], and development of “pseudoantigens” [22].
The osmolality of the contrast medium as well as the size and complexity of the molecule has potential influence on the
likelihood of contrast reactions. Hyperosmolality is associated with stimulation of histamine release from basophils and mast
cells. Increase in the size and complexity of the contrast molecule may potentiate the release of histamine [23,24]. There is
some evidence to suggest that low-osmolality nonionic monomers produce lower levels of histamine release from basophils
compared with high-osmolality ionic monomers, low-osmolality ionic dimers and iso-osmolality nonionic dimers [24]. Low-
osmolality monomeric contrast media also are associated with a reduced likelihood of physiologic reactions following
intravenous administration (i.e., non-allergic-like; e.g., nausea and vomiting). In general, non-ionic iodinated contrast media
are associated with less adverse events than ionic contrast media (iodinated and gadolinium- based) [2,25].
Benefits of Premedication: A randomized trial showed that premedication of average-risk patients prior to high- osmolality
iodinated contrast medium administration reduces the likelihood of immediate adverse events of all severity [21]. However,
high-osmolality contrast medium is no longer used for intravascular purposes.
Another randomized trial showed that premedication of average-risk patients prior to modern low- osmolality iodinated contrast
medium administration reduce the likelihood of mild and aggregate immediate adverse events, but the trial was underpowered
to evaluate the effect on moderate and severe reactions [26].
Both of these randomized trials of premedication did not study the effect of premedication in high-risk patients who are
usually premedicated today, and neither study was sufficiently powered to evaluate the efficacy of premedication in the
prevention of moderate or severe reactions [21,26].
Nonetheless, many experts believe that premedication does reduce the likelihood of a reaction in high- risk patients receiving
low-osmolality iodinated contrast medium [26], although the number needed to treat to prevent a reaction is high [27,28].
One study estimated that the number needed to premedicate to prevent one reaction in high-risk patients was 69 for a reaction
of any severity and 569 for a severe reaction [27]. Another study estimated the number needed to treat to prevent a lethal
reaction in high-risk patients to be 50,000 [28].
PATIENT SELECTION AND PREPARATION STRATEGIES BEFORE CONTRAST MEDIUM ADMINISTRATION 8
There are no studies evaluating the efficacy of premedication prior to oral contrast medium administration or gadolinium-
based contrast medium administration in high-risk patients. Premedication strategies in these patients are based on
extrapolated data from patients receiving intravascular iodinated media.
Risks of Premedication: The direct risks of premedication are small [29] and include transient leukocytosis, transient (24-
48h) and usually asymptomatic hyperglycemia (non-diabetics: +20-80 mg/dL, diabetics: +100-150 mg/dL) [30,31], and a
questionable infection risk, among other things. Diphenhydramine may cause drowsiness and should not be taken shortly
before operating a vehicle. Some patients have experienced allergies to the individual medications used in premedication.
The largest risk of premedication is indirect and related to the delay in diagnosis imparted by the multi- hour duration of
premedication [30]. In one retrospective cohort study of 2829 subjects, 13-hour oral premedication of high-risk inpatients was
associated with increased hospital length of stay (median: +25h), increased time to CT (median: +25h), increased hospital-
acquired infection risk, and increased costs compared to non-premedicated controls [30]. The indirect harms of premedication
likely overshadow the benefits of premedication in some vulnerable populations.
Breakthrough Contrast Reactions: Premedication does not prevent all contrast reactions [27,32,33]. Allergic- like contrast
reactions that occur despite premedication are called “breakthrough reactions” [32]. Physiologic reactions are not mitigated
by premedication and are not considered “breakthrough reactions,” even if they occur following premedication.
Patients premedicated for a prior contrast reaction have a breakthrough reaction rate (2.1%) that is 3-4 times the ordinary
reaction rate in the general population, while patients premedicated for other indications have a breakthrough reaction rate
close to 0% [27]. In most cases (~81%), breakthrough reaction severity is similar to index reaction severity [32,33]. Patients
with a mild index reaction have a very low risk (<1%) of developing a severe breakthrough reaction [27].
The majority (~88%) of contrast injections in premedicated patients with a prior breakthrough reaction will not result in a
repeat breakthrough reaction [32,33]. Repeat breakthrough reactions, if they occur, usually are of similar severity to prior
breakthrough reactions. Therefore, patients who have had a prior moderate or severe breakthrough reaction are at the highest
risk for developing a future moderate or severe breakthrough reaction [32,33].
Premedication Strategies: Oral premedication is preferable to IV premedication in most settings due to lower cost, more
convenience, and greater evidentiary support in the literature [21,26]. The randomized trials of premedication in average-risk
patients were conducted with oral methylprednisolone [21,26]. Uncontrolled studies in high-risk patients were conducted with
oral prednisone [34,35].
Supplemental administration of a non-selective antihistamine (e.g., diphenhydramine) orally or intravenously 1 hour prior to
contrast medium administration may reduce the frequency of urticaria, angioedema, and respiratory symptoms. Use of selective
anti-histamines (i.e., selective H2 blockers) has not been well studied [34].
The minimum duration of premedication necessary for efficacy is unknown. Lasser et al [26] showed that one dose of 32 mg
oral methylprednisolone 2 hours prior to IV high-osmolality iodinated contrast medium administration in average-risk patients
was not effective, while two doses administered at 2- and 12-hours before contrast medium administration were effective
[26].
A dose-response study of single-dose IV methylprednisolone (1 mg/kg) [36] in 11 volunteers showed a reduction in circulating
basophils and eosinophils by the end of the first post-injection hour, reaching statistical significance compared with controls
by the end of the second hour and a concomitant reduction in histamine in sedimented leukocytes by 4 hours. Most of these
effects reached their peak at 8 hours.
There is no evidence to support a premedication duration of 2 hours or less (oral or IV; corticosteroid- or antihistamine-
based).
An IV corticosteroid regimen with a minimum duration of 4-5 hours may be efficacious [10,26,29,36].
PATIENT SELECTION AND PREPARATION STRATEGIES BEFORE CONTRAST MEDIUM ADMINISTRATION 9
Indications for Premedication
Given that premedication does not prevent all reactions, has not been confirmed to reduce the incidence of moderate or severe
reactions or reaction-related deaths, has limited supporting efficacy in high-risk patients, and is accompanied by direct and
indirect harms, the utility of premedication in high- risk patients is uncertain. Given the tradeoffs between what is known and
not known with respect to the benefits and harms of premedication, premedication may be considered in the following settings
and scenarios:
12- or 13-hour oral premedication maybe considered in the following settings:
1.
Outpatient with a prior allergic-like or unknown-type contrast reaction to the same class of contrast medium (e.g.,
iodinated – iodinated).
2.
Emergency department patient or inpatient with a prior allergic-like or unknown-type contrast reaction to the same
class of contrast medium (e.g., iodinated – iodinated) in whom the use of premedication is not anticipated to
adversely delay care decisions or treatment.
Accelerated IV premedication may be considered in the following settings:
1.
Outpatient with a prior allergic-like or unknown-type contrast reaction to the same class of contrast medium (e.g.,
iodinated – iodinated) who has arrived for a contrast-enhanced examination but has not been premedicated and
whose examination cannot be easily rescheduled.
2.
Emergency department patient or inpatient with a prior allergic-like or unknown-type contrast reaction to the same
class of contrast medium (e.g., iodinated – iodinated) in whom the use of 12- or 13-hour premedication is anticipated
to adversely delay care decisions or treatment.
In rare clinical situations, the urgency of a contrast-enhanced examination may outweigh the benefits of prophylaxis,
regardless of duration, necessitating that contrast medium be administered to a high-risk patient in the absence of
premedication. This determination is best made jointly by the radiology team, the referring service, and potentially the patient
(if feasible). In such cases, a team of individuals skilled in resuscitation should be available during the injection to monitor
for and appropriately manage any developing reaction.
Regardless of patient status, history of a prior severe contrast reaction is considered a relative contraindication to receiving
the same class of contrast medium in the future. If the same class of contrast medium is necessary and there are no alternatives,
premedication should be considered, if feasible.
Routine premedication or avoidance of contrast medium for other indications, such as allergic reactions to other substances
(including shellfish or contrast media from another class [e.g., gadolinium-based – iodinated]), asthma, seasonal allergies, or
multiple drug and food allergies is not recommended.
Specific Recommended Premedication Regimens
Elective Premedication (12- or 13-hour oral premedication)
1.
Prednisone-based: 50 mg prednisone by mouth at 13 hours, 7 hours, and 1 hour before contrast medium
administration, plus 50 mg diphenhydramine intravenously, intramuscularly, or by mouth 1 hour before contrast
medium administration [21].
Or
2.
Methylprednisolone-based: 32 mg methylprednisolone by mouth 12 hours and 2 hours before contrast medium
administration. 50 mg diphenhydramine may be added as in option 1 [37].
Although never formally compared, both regimens are considered similarly effective. The presence of
diphenhydramine in regimen 1 and not in regimen 2 is historical and not evidence-based. Therefore,
PATIENT SELECTION AND PREPARATION STRATEGIES BEFORE CONTRAST MEDIUM ADMINISTRATION 10
diphenhydramine may be considered optional.
If a patient is unable to take oral medication, option 1 may be used substituting 200 mg hydrocortisone IV for each dose of oral
prednisone [38]. If a patient is allergic to diphenhydramine in a situation where diphenhydramine would otherwise be
considered, an alternate anti-histamine without cross-reactivity may be considered, or the anti-histamine portion of the regimen
may be dropped.
Accelerated IV Premedication (in decreasing order of desirability)
1.
Methylprednisolone sodium succinate (e.g., Solu-Medrol
®
) 40 mg IV or hydrocortisone sodium succinate (e.g., Solu-
Cortef
®
) 200 mg IV immediately, and then every 4 hours until contrast medium administration, plus
diphenhydramine 50 mg IV 1 hour before contrast medium administration. This regimen usually is 4-5 hours in
duration.
2.
Dexamethasone sodium phosphate (e.g., Decadron
®
) 7.5 mg IV immediately, and then every 4 hours until contrast
medium administration, plus diphenhydramine 50 mg IV 1 hour before contrast medium administration. This
regimen may be useful in patients with an allergy to methylprednisolone and is also usually 4-5 hours in duration.
3.
Methylprednisolone sodium succinate (e.g., Solu-Medrol
®
) 40 mg IV or hydrocortisone sodium succinate (e.g., Solu-
Cortef
®
) 200 mg IV, plus diphenhydramine 50 mg IV, each 1 hour before contrast medium administration. This
regimen, and all other regimens with a duration less than 4-5 hours, has no evidence of efficacy. It may be considered
in emergent situations when there are no alternatives.
Note: Premedication regimens less than 4-5 hours in duration (oral or IV) have not been shown to be effective. The
accelerated 4-5-hour regimen listed as Accelerated IV option 1 is supported by a case series and by a retrospective
cohort study with 828 subjects [38].
Missing One or More Doses of Premedication
Sometimes, patients undergoing premedication present for a contrast-enhanced scan without completing their premedication
regimen. In such cases, there is no evidence base to guide decision-making, so management should be individualized.
Generally speaking, if premedication is being used, a guiding principle is to have a minimum of 4-5 hours of corticosteroid
therapy prior to contrast medium exposure, with repeat doses every 4-8 hours. Diphenhydramine administration is optional.
Premedication in Patients Undergoing Chronic Corticosteroid Therapy
In patients who have had a prior allergic-like reaction to contrast medium and who are also on chronic corticosteroid therapy,
premedication dosing may be modified. In this circumstance, there is no evidence base to guide decision-making, so
management should be individualized. Generally speaking, if corticosteroid premedication is being used, a guiding principle
is to reduce the dose of the chosen premedication dose regimen by an amount equivalent to the patient’s chronic therapeutic
corticosteroid dose. If the patient is on simple replacement (not therapeutic) corticosteroids, the premedication dosing regimen
may not need to be adjusted.
Changing Contrast Media Within the Same Class
In patients with a prior allergic-like or unknown-type contrast reaction to a known contrast medium, changing contrast media
within the same class (e.g., one iodinated medium for another) may help reduce the likelihood of a subsequent contrast reaction
[39,40]. Some studies have shown that the effect size of switching contrast media actually may be greater than that of
premedication alone, but combining premedication with a change in agent seems to have the greatest effect [39,40].
Unfortunately, many patients do not know which specific agent they have reacted to in the past; they simply remember they
had a reaction. In the future, through improved electronic medical records, routine linking of reactions to specific contrast
media is likely to add value. In the current state, investigating which agent was responsible for one or more prior reactions
often is not possible.

PATIENT SELECTION AND PREPARATION STRATEGIES BEFORE CONTRAST MEDIUM ADMINISTRATION 11
Premedication Is Not a Panacea
No premedication strategy is a substitute for pre-administration preparedness. Contrast reactions occur despite premedication
[32], and radiology teams must be prepared to treat breakthrough reactions when they occur. Patients should receive
information concerning their risk of a reaction according to local policy and practice.
REFERENCES
1.
Wang CL, Cohan RH, Ellis JH, Caoili EM, Wang G, Francis IR. Frequency, outcome, and appropriateness of treatment of nonionic iodinated
contrast media reactions. AJR Am J Roentgenol 2008;191:409-15.
2.
Katayama H, Yamaguchi K, Kozuka T, Takashima T, Seez P, Matsuura K. Adverse reactions to ionic and nonionic contrast media. A report from
the Japanese Committee on the Safety of Contrast Media. Radiology 1990;175:621-8.
3.
Jung JW, Kang HR, Kim MH, et al. Immediate hypersensitivity reaction to gadolinium-based MR contrast media. Radiology 2012;264:414-22.
4.
Beaty AD, Lieberman PL, Slavin RG. Seafood allergy and radiocontrast media: are physicians propagating a myth? Am J Med 2008;121:158.e1-
4.
5.
Boehm I. Seafood allergy and radiocontrast media: are physicians propagating a myth? Am J Med 2008;121:e19.
6.
Shehadi WH. Adverse reactions to intravascularly administered contrast media. A comprehensive study based on a prospective su rvey. Am J
Roentgenol Radium Ther Nucl Med 1975;124:145-52.
7.
Lalli AF. Urographic contrast media reactions and anxiety. Radiology 1974;112:267-71.
8.
Callahan MJ, Poznauskis L, Zurakowski D, Taylor GA. Nonionic iodinated intravenous contrast material-related reactions: incidence in large
urban children's hospital--retrospective analysis of data in 12,494 patients. Radiology 2009;250:674-81.
9.
Lang DM, Alpern MB, Visintainer PF, Smith ST. Elevated risk of anaphylactoid reaction from radiographic contrast media is associated with
both beta-blocker exposure and cardiovascular disorders. Arch Intern Med 1993;153:2033-40.
10.
Morcos SK. Review article: Acute serious and fatal reactions to contrast media: our current understanding. Br J Radiol 2005;78:686-93.
11.
Mukherjee JJ, Peppercorn PD, Reznek RH, et al. Pheochromocytoma: effect of nonionic contrast medium in CT on circulating catecholamine
levels. Radiology 1997;202:227-31.
12.
Mehrizi M, Pascuzzi RM. Complications of radiologic contrast in patients with myasthenia gravis. Muscle Nerve 2014;50:443-4.
13.
Somashekar DK, Davenport MS, Cohan RH, Dillman JR, Ellis JH. Effect of intravenous low-osmolality iodinated contrast media on patients
with myasthenia gravis. Radiology 2013;267:727-34.
14.
van der Molen AJ, Thomsen HS, Morcos SK. Effect of iodinated contrast media on thyroid function in adults. Eur Radiol 2004;14:902-7.
15.
Silberstein EB, Alavi A, Balon HR, et al. The SNMMI practice guideline for therapy of thyroid disease with 131I 3.0. J Nucl M ed 2012;53:1633-
51.
16.
Society of Nuclear Medicine. Therapy of Thyroid Disease with Iodine-131 v2.0. Available at:
http://interactive.snm.org/docs/Therapy%20of%20Thyroid%20Disease%20with%20Iodine-131%20v2.0.pdf.
17.
Palena LM, Sacco ZD, Brigato C, et al. Discomfort assessment in peripheral angiography: randomized clinical trial of Iodixanol 270 versus
Ioversol 320 in diabetics with critical limb ischemia. Catheter Cardiovasc Interv 2014;84:1019-25.
18.
Laroche D, Aimone-Gastin I, Dubois F, et al. Mechanisms of severe, immediate reactions to iodinated contrast material. Radiology
1998;209:183-90.
19.
Trcka J, Schmidt C, Seitz CS, Bröcker EB, Gross GE, Trautmann A. Anaphylaxis to iodinated contrast material: nonallergic hypersensitivity or
IgE-mediated allergy? AJR Am J Roentgenol 2008;190:666-70.
20.
Yamaguchi K, Katayama H, Takashima T, Kozuka T, Seez P, Matsuura K. Prediction of severe adverse reactions to ionic and nonionic contrast
media in Japan: evaluation of pretesting. A report from the Japanese Committee on the Safety of Contrast Media. Radio logy 1991;178:363-7.
21.
Lasser EC, Berry CC, Talner LB, et al. Pretreatment with corticosteroids to alleviate reactions to intravenous contrast material. N Engl J Med
1987;317:845-9.
22.
Lasser EC. The multipotential pseudoantigenicity of X-ray contrast media. Pseudoantigen excess may downregulate the release of hypotensive
mediators. Int Arch Allergy Immunol 2000;123:282-90.
23.
Paton WD. Histamine release by compounds of simple chemical structure. Pharmacol Rev 1957;9:269-328.
24.
Peachell PT, Morcos SK. Effect of radiographic contrast media on histamine release from human mast cells and basophils. Br J Radiol
1998;71:24-30.
25.
Lasser EC, Berry CC, Mishkin MM, Williamson B, Zheutlin N, Silverman JM. Pretreatment with corticosteroids to prevent adverse reactions to
nonionic contrast media. AJR Am J Roentgenol 1994;162:523-6.
26.
O'Malley RB, Cohan RH, Ellis JH, et al. A survey on the use of premedication prior to iodinated and gadolinium -based contrast material
administration. J Am Coll Radiol 2011;8:345-54.
27.
Mervak BM, Davenport MS, Ellis JH, Cohan RH. Rates of Breakthrough Reactions in Inpatients at High Risk Receiving Premedication Before
Contrast-Enhanced CT. AJR Am J Roentgenol 2015;205:77-84.
28.
Davenport MS, Mervak BM, Ellis JH, Dillman JR, Dunnick NR, Cohan RH. Indirect Cost and Harm Attributable to Oral 13-Hour Inpatient
Corticosteroid Prophylaxis before Contrast-enhanced CT. Radiology 2016;279:492-501.
29.
Lasser EC. Pretreatment with corticosteroids to prevent reactions to i.v. contrast material: overview and implications. AJR Am J Roentgenol
1988;150:257-9.
30.
Davenport MS, Cohan RH, Caoili EM, Ellis JH. Hyperglycemic consequences of corticosteroid premedication in an outpatient popu lation. AJR
Am J Roentgenol 2010;194:W483-8.
31.
Davenport MS, Cohan RH, Khalatbari S, Myles J, Caoili EM, Ellis JH. Hyperglycemia in hospitalized patients receiving corticosteroid
premedication before the administration of radiologic contrast medium. Acad Radiol 2011;18:384-90.
32.
Freed KS, Leder RA, Alexander C, DeLong DM, Kliewer MA. Breakthrough adverse reactions to low-osmolar contrast media after steroid
premedication. AJR Am J Roentgenol 2001;176:1389-92.
33.
Davenport MS, Cohan RH, Caoili EM, Ellis JH. Repeat contrast medium reactions in premedicated patients: frequency and severit y. Radiology
2009;253:372-9.
PATIENT SELECTION AND PREPARATION STRATEGIES BEFORE CONTRAST MEDIUM ADMINISTRATION 12
34.
Greenberger PA, Patterson R, Tapio CM. Prophylaxis against repeated radiocontrast media reactions in 857 cases. Adverse exper ience with
cimetidine and safety of beta-adrenergic antagonists. Arch Intern Med 1985;145:2197-200.
35.
Greenberger PA, Patterson R, Radin RC. Two pretreatment regimens for high -risk patients receiving radiographic contrast media. J Allergy Clin
Immunol 1984;74:540-3.
36.
Dunsky EH, Zweiman B, Fischler E, Levy DA. Early effects of corticosteroids on basophils, leukocyte histamine, and tissue histamine. J Allergy
Clin Immunol 1979;63:426-32.
37.
Greenberger PA, Patterson R. The prevention of immediate generalized reactions to radiocontrast media in high-risk patients. J Allergy Clin
Immunol 1991;87:867-72.
38.
Mervak BM, Cohan RH, Ellis JH, Khalatbari S, Davenport MS. Intravenous Corticosteroid Premedication Administered 5 Hours befo re CT
Compared with a Traditional 13-Hour Oral Regimen. Radiology 2017;285:425-33.
39.
Abe S, Fukuda H, Tobe K, Ibukuro K. Protective effect against repeat adverse reactions to iodinated contrast medium: Premedication vs.
changing the contrast medium. Eur Radiol 2016;26:2148-54.
40.
Park HJ, Park JW, Yang MS, et al. Re-exposure to low osmolar iodinated contrast media in patients with prior moderate-to-severe
hypersensitivity reactions: A multicentre retrospective cohort study. Eur Radiol 2017;27:2886-93.
FASTING PRIOR TO INTRAVASCULAR CONTRAST MEDIA ADMINISTRATION 13
Fasting Prior to Intravascular Contrast Media Administration
To decrease the likelihood of vomiting and aspiration, some practices request that patients fast prior to administration on
intravenous contrast media [1]. However, currently used low- and iso-osmolality nonionic iodinated contrast media used for
CT, and gadolinium-based contrast media (GBCM) used for MRI, have much lower risk of vomiting compared to the
previously used ionic high-osmolality iodinated contrast media [2-9]. Furthermore, pre-procedure fasting may have negative
effects including scheduling limitations, hypoglycemic risk in patients with diabetes mellitus, and general discomfort
A 2012 meta-analysis of 13 studies and 2,001 patients exposed to intravascular iodinated contrast media found that, despite
heterogeneous fasting practices (including many with no restrictions), there were no cases of aspiration pneumonia attributab le
to iodinated contrast media [1]. A 2009 study of 158,439 injections of GBCM demonstrated that only 0.03% resulted in mild
adverse events including nausea, vomiting, or mild rash [10]. No assessment of the risk aspiration pneumonia attributable to
GBCM has been performed. Data indicate there is no preventive effect of fasting prior to modern iodinated and gadolinium-
based intravascular contrast media administration on risk of nausea, vomiting, or aspiration [11].
Given the potential for negative consequences due to fasting and a lack of evidence that supports the need for fasting, fasting is
not required prior to routine intravascular contrast material administration. However, for patients receiving conscious sedation,
anesthesia guidelines should be consulted (e.g., the American Society of Anesthesiology [ASA] Practice Guidelines for
Preoperative Fasting in Health Patients Undergoing Elective Procedures) [12].
References
1. Lee BY, Ok JJ, Abdelaziz Elsayed AA, Kim Y, Han DH. Preparative fasting for contrast-enhanced CT: reconsideration. Radiology.
2012;263(2):444-450.
2. Bush WH, Swanson DP. Acute reactions to intravascular contrast media: types, risk factors, recognition, and specific treatmen t. AJR Am J
Roentgenol. 1991;157(6):1153-1161.
3. Katayama H, Yamaguchi K, Kozuka T, Takashima T, Seez P, Matsuura K. Adverse reactions to ionic and nonionic contrast media. A report
from the Japanese Committee on the Safety of Contrast Media. Radiology. 1990;175(3):621-628.
4. Murphy KJ, Brunberg JA, Cohan RH. Adverse reactions to gadolinium contrast media: a review of 36 cases. AJR Am J Roentgenol.
1996;167(4):847-849.
5. Runge VM. Safety of approved MR contrast media for intravenous injection. J Magn Reson Imaging. 2000;12(2):205-213.
6. Runge VM. Safety of magnetic resonance contrast media. Top Magn Reson Imaging. 2001;12(4):309-314.
7. Murphy KP, Szopinski KT, Cohan RH, Mermillod B, Ellis JH. Occurrence of adverse reactions to gadolinium -based contrast material and
management of patients at increased risk: a survey of the American Society of Neuroradiology Fellowship Directors. Acad Radiol.
1999;6(11):656-664.
8. Barbosa P, Bitencourt AGV, Tyng CJ, et al. JOURNAL CLUB: Preparative Fasting for Contrast-Enhanced CT in a Cancer Center: A New
Approach. AJR Am J Roentgenol. 2018;210(5):941-947.
9. Wagner HJ, Evers JP, Hoppe M, Klose KJ. [Must the patient fast before intravascular injection of a non -ionic contrast medium? Results of a
controlled study]. Rofo. 1997;166(5):370-375.
10. Hunt CH, Hartman RP, Hesley GK. Frequency and severity of adverse effects of iodinated and gadolinium contrast materials: ret rospective
review of 456,930 doses. AJR Am J Roentgenol. 2009;193(4):1124-1127.
11. Neeman Z, Abu Ata M, Touma E, et al. Is fasting still necessary prior to contrast-enhanced computed tomography? A randomized clinical
study. Eur Radiol. 2020.
12. Anonymous. Practice Guidelines for Preoperative Fasting and the Use of Pharmacologic Agents to Reduce the Risk of Pulmonary Aspiration:
Application to Healthy Patients Undergoing Elective Procedures: An Updated Report by the American Society of Anesthesiologists Task Force
on Preoperative Fasting and the Use of Pharmacologic Agents to Reduce the Risk of Pulmonary Aspiration*. Anesthesiology. 2017;126(3):376-
393.

SAFE INJECTION OF CONTRAST MEDIA 14
SAFE INJECTION OF CONTRAST MEDIA
General Considerations
Injection methods vary depending on vascular access, differential diagnosis, and imaging examination type. The mode and
method of delivery, either by hand or by power injector, also vary by procedure. Subject to the requirements of state law, a
radiologist, radiologic technologist, or nurse may administer contrast media. Stable intravenous (IV) access is necessary. For
current American College of Radiology (ACR) recommendations regarding injection of contrast media (including
radiopharmaceuticals), see the ACR–SPR Practice Parameter for the Use of Intravascular Contrast Media.
Referring to FDA package inserts may be appropriate in determining contrast media doses and concentrations (see Appendix
A – Contrast Media Specifications). It is important to avoid prolonged admixture of blood and contrast media in syringes and
catheters whenever possible due to the risk of clot formation. In general, unless known to be safe, the admixture of contrast
media and any medication should be avoided. However, heparin may be combined with contrast media.
Mechanical Injection of Intravenous Contrast Media
Bolus or power injection of IV contrast material is superior to drip infusion for enhancing normal and abnormal structures
during body computed tomography (CT). Radiology personnel must recognize the need for proper technique to avoid the
potentially serious complications of contrast media extravasation and air embolism. (See the Chapter on Extravasation of
Contrast Media). When proper technique is used, contrast medium can be safely administered intravenously by power injector
in the vast majority of patients, even at high-flow rates.
Technique
To avoid potential complications, the patient’s cooperation should be obtained whenever possible. Communicating with the
patient before the examination and during the injection may reduce the risk of contrast medium extravasation. If the patient
reports pain or the sensation of swelling at the injection site, injection should be discontinued.
Intravenous contrast media should be administered by power injector through a flexible plastic cannula. Use of metal needles
for power injection should be avoided whenever possible. In addition, the flow rate should be appropriate for the gauge of the
catheter used. Although 22-gauge catheters may be able to tolerate flow rates up to 5 ml/sec, a 20-gauge or larger catheter is
preferable for flow rates of 3 ml/sec or greater. An antecubital or large forearm vein is the preferred venous access site for
power injection. If a more peripheral (e.g., hand or wrist) venipuncture site must be used, flow rates should be reduced if
feasible (e.g., 1-2 mL/sec).
Careful preparation of the power injection apparatus is essential to minimize the risk of contrast medium extravasation or air
embolism. Standard procedures should be used to clear the syringe and pressure tubing of air, after which the syringe should
be reoriented with the tubing directed downward. Several maneuvers can be performed to confirm the proper intravenous
location of an inserted catheter. The catheter to be used can be checked for backflow of blood into the tubing, although
backflow is not always noted, even in an appropriately positioned intravenous line. A saline test flush can be performed by
hand or once the tubing is connected to a power injector. Direct monitoring of the site during injection can be performed if
feasible, but direct monitoring often is not feasible, particularly when CT arteriography is performed or when automatic
triggering programs are employed. If the venipuncture site is found to be tender or infiltrated during any of these maneuvers,
an alternative site should be sought. In all instances, the power injector and its tubing should be positioned to allow adequate
table movement without tension on the intravenous line.
A means of easy communication between the technologist and the patient is required at all times prior to, during, and following
a contrast media injection. This initially can occur via direct contact and then by use of an intercom or television system.
When feasible, the patient should be notified of the presence of such a system and instructed to notify the technologist for any
changes in sensation, including increasing pain or swelling at the injection site.
It should not be assumed that power injection can be performed in all central venous catheters. However, power injection of
contrast media through some central venous catheters can be performed safely provided that certain precautions are followed.
Before connecting the catheter to the injector system tubing, the catheter tip position should be tested for venous backflow.
Occasionally backflow will not be obtained because the catheter tip is positioned against the wall of the vein in which it is
located. If saline can be injected through the catheter without abnormal resistance, contrast media can be administered through
the catheter safely. If abnormal resistance or discomfort is encountered, an alternative venous access site should be sought.
Injection with large-bore (9.5-F to 10-F) central venous catheters using flow rates of up to 2.5 ml/ sec has been shown to
SAFE INJECTION OF CONTRAST MEDIA 15
generate pressures below manufacturers’ specified limits. For power injection of contrast media through some central venous
catheters, the radiologist should consult manufacturers’ recommendations. Contrast media should not be administered by
power injector through small-bore, peripheral (e.g., arm) access central venous catheters unless permitted by the
manufacturer’s specifications because of the risk of catheter breakage. Such catheters will usually have a specific rating th at
indicates they can be used for power injection up to a specified flow rate.
Air Embolism
Clinically significant large-volume venous air embolism is a potentially fatal but rare complication of IV contrast media
injection. However, small-volume clinically insignificant venous air embolism commonly occurs. Using care when using
power injection for contrast-enhanced CT minimizes the risk of clinically significant air embolism. On CT, venous air
embolism is most commonly identified as air bubbles or air-fluid levels in the intrathoracic veins, main pulmonary artery, or
right ventricle, although it can conceivably be visualized in any vessel downstream of the injection (e.g, intracranial veins).
Inadvertent injection of large amounts of air into the venous system may result in air hunger, dyspnea, cough, chest pain,
pulmonary edema, tachycardia, hypotension, and expiratory wheezing. Neurologic deficits may result from stroke due to
decreased cardiac output or paradoxical air embolism. Patients with right-to-left intracardiac shunts or pulmonary
arteriovenous malformations are at a higher risk of having a neurological deficit develop from small volumes of air embolism.
Treatment of venous air embolism includes administration of 100% oxygen and placing the patient in the left lateral decubitus
position (i.e., left side down). Hyperbaric oxygen has been recommended to reduce the size of air bubbles and to restore
circulation and oxygenation. If cardio-pulmonary arrest occurs, closed-chest cardiopulmonary resuscitation should be initiated
immediately.
Intra-osseous Injection
Intra-osseous (IO) catheters allow rapid intravascular access for the administration of fluids and medications in critically ill
patients without intravenous access. Over the last two decades, there have been improvements in product design and speed
of line placement that have translated into a low reported complication rate [1-3]. Three common devices on the market in
the United States include: The Bone Insertion Gun (BIG) (WaisMed, Israel); the First Access in Shock and Trauma (FAST1)
(Pyng Medical Corporation, Richmond, Canada); and the EZ-IO (Vidacare, San Antonio, USA), which uses a battery-
powered driver (similar to a hand-held drill) to place the specially designed needle [1,2]. Humeral placement is now the
preferred site of access secondary to quick line placement and higher achievable flow rates compared to tibial access [1,4,5].
High pressures are needed to infuse through IO lines because of high intramedullary compartmental pressures. Power
injection is possible for CT and MRI; however, the rates for injection and pressure settings are not well studied in humans.
While no large studies looking at IO access for administration of contrast media exist, several case reports document
successful acquisition of contrast-enhanced CT with no reported complications using injection rates up to 5 ml/sec (max PSI
of 300) [4,6-9]. Intra-osseous injection of gadolinium-based contrast media has not been studied, but there is no reason to
believe it would behave differently.
A local anesthetic is needed in non-sedated patients prior to infusion of any substance through IO access. A few small studies
have looked at different lidocaine algorithms to minimize the pain of infusion [1,5,10]. One suggested pretreatment reported
from a single institution with the EZ-IO device is 40 mg 2% (2 ml) of epinephrine-free lidocaine slowly infused over 2
minutes after the line is primed with 1 ml lidocaine. The medication was allowed to dwell for one minute, and then the line
was flushed with 5-10 ml of saline followed by another 20 mg (1 ml) of lidocaine infused over one minute. For pediatric
patients the same algorithm would be used, with 0.5 mg/kg as the initial dose (not to exceed 40 mg), followed by a 2-5 ml
saline flush and a second 0.25 mg/kg lidocaine dose [4]. If a radiology practice is not familiar with IO infusions, consult the
local trauma team for advice on how and whether to prime the line with anesthetic using local protocols.
Revision History
21 December 2017: Minor revision
10 June 2016: Major revision
29 October 2008: Major revisions
8 March 2004 (First version)
References
1.
Sum W, Ridley LJ. Recognition and management of contrast media extravasation. Australas Radiol. 2006; 50(6):549-552.
2.
Park KS, Kim SH, Park JH, Han MC, Kim DY, Kim SJ. Methods for mitigating soft-tissue injury after subcutaneous injection of water-soluble
contrast media. Invest Radiol. 1993;28(4):332-334.
3.
Cohan RH, Leder RA, Herzberg AJ, et al. Treatment of injuries produced by extravasation of radiographic contrast media. Presented at the 39th
SAFE INJECTION OF CONTRAST MEDIA 16
Annual Meeting of the Association of University Radiologists. 1990; Minneapolis, MN.
4.
Rowlett J. Extravasation of contrast media managed with recombinant human hyaluronidase. Am J Emerg Med. 2012;30(9):2102 e2101-2103.
5.
Hanrahan K. Hyaluronidase for treatment of intravenous extravasations: implementation of an evidence-based guideline in a pediatric population.
J Spec Pediatr Nurs. 2013;18(3):253-262.
6.
Berson EL. Experimental and therapeutic aspects of photic damage to the retina. Invest Ophthalmol. 1973;12(1):35-44.
7.
Upton J, Mulliken JB, Murray JE. Major intravenous extravasation injuries. Am J Surg. 1979;137(4):497-506.
8.
Lewis GB, Hecker JF. Radiological examination of failure of intravenous infusions. Br J Surg. 1991;78(4):500-501.
9.
Wei K, Mulvagh SL, Carson L, et al. The safety of deFinity and Optison for ultrasound image enhancement: a retrospective analysis of 78,383
administered contrast doses. J Am Soc Echocardiogr. 2008;21(11):1202-1206.
10.
Piscaglia F, Bolondi L, Italian Society for Ultrasound in M, Biology Study Group on Ultrasound Contrast A. The safety of Sono vue in abdominal
applications: retrospective analysis of 23188 investigations. Ultrasound Med Biol. 2006;32(9):1369-1375.
Suggested Reading (Articles that the Committee recommends for further reading on this topic are provided here).
1.
Carlson JE, Hedlund LJ, Trenkner SW, Ritenour R, Halvorsen RA, Jr. Safety considerations in the power injection of contrast m edia via central
venous catheters during computed tomographic examinations. Invest Radiol 1992; 27:337-340.
2.
Coyle D, Bloomgarden D, Beres R, Patel S, Sane S, Hurst E. Power injection of contrast media via peripherally inserted central catheters for
CT. J Vasc Interv Radiol 2004; 15:809-814.
3.
Herts BR, Cohen MA, McInroy B, Davros WJ, Zepp RC, Einstein DM. Power injection of intravenous contrast material through cent ral venous
catheters for CT: in vitro evaluation. Radiology 1996; 200:731-735.
4.
Kizer KW, Goodman PC. Radiographic manifestations of venous air embolism. Radiology 1982; 144:35-39.
5.
McCarthy S, Moss AA. The use of a flow rate injector for contrast-enhanced computed tomography. Radiology 1984; 151:800.
6.
Murphy BP, Harford FJ, Cramer FS. Cerebral air embolism resulting from invasive medical procedures. Treatment with hyperbaric oxygen.
Ann Surg 1985; 201:242-245.
7.
Price DB, Nardi P, Teitcher J. Venous air embolization as a complication of pressure injection of contrast media: CT findings.
J Comput Assist Tomogr 1987; 11:294-295.
8.
Rubinstein D, Dangleis K, Damiano TR. Venous air emboli identified on head and neck CT scans. J Comput Assist Tomogr
1996; 20:559-562.
9.
Ruess L, Bulas DI, Rivera O, Markle BM. In-line pressures generated in small-bore central venous catheters during power injection of CT
contrast media. Radiology 1997; 203:625-629.
10.
Shuman WP, Adam JL, Schoenecker SA, Tazioli PR, Moss AA. Use of a power injector during dynamic computed tomography.
J Comput Assist Tomogr 1986; 10:1000-1002.
11.
Williamson EE, McKinney JM. Assessing the adequacy of peripherally inserted central catheters for power injection of intravenous contrast
agents for CT. J Comput Assist Tomogr 2001; 25:932-937.
12.
Woodring JH, Fried AM. Nonfatal venous air embolism after contrast-enhanced CT. Radiology 1988; 167:405-407.

EXTRAVASATION OF CONTRAST MEDIA 17
Extravasation Bullet Points and Recommendations with Associated Strength of Evidence
Below is a summary of the recommendations related to contrast extravasation and the associated strength of evidence for
those recommendations using the ACR Appropriateness Criteria® Methodology.
Frequency
•
0.1-1.2% of CT injections result in extravasations [1-9].
•
Most extravasations resolve without complication [6, 7, 11, 13, 14]; severe extravasation injuries, including
compartment syndrome (most common [11]) and skin ulceration / necrosis, are very rare (<<1% of
extravasations) [7, 8, 11].
Risks
•
Extravasations and severe extravasation injuries are more common in patients who 1) are uncommunicative, 2) have
altered circulation in the injected extremity, 3) have had radiation of the injected extremity, or 4) are injected in the hand,
foot, or ankle [1, 32].
•
Extravasations are also more common in patients injected with more viscous contrast material [6, 8, 35].
•
The risk of extravasation can be minimized by 1) using angiocatheters rather than butterfly needles, 2) performing
meticulous intravenous catheter insertion technique (confirming intravenous location by aspirating blood through an
inserted catheter and flushing the inserted catheter with a test injection), 3) and carefully securing an inserted catheter
[41].
Evaluation and treatment
•
A health care provider should examine any patient in whom a contrast-media extravasation occurs; physical
examination should include assessment of tenderness, swelling, erythema, paresthesia, active and passive range of
finger motion, and perfusion [22].
•
There is no known effective treatment for contrast medium extravasation, although initial steps should include
elevation of the affected extremity above the level or the heart [22, 24], and use of cold or warm compresses [22-
25]. No medical interventions have been deemed helpful [15, 22, 28, 29].
•
Since severe extravasation injuries can develop slowly (up to hours after an extravasation), all discharged
outpatients should be given clear instructions concerning where and when to seek additional medical care
(including for worsening pain, development of paresthesia, diminished range of motion, and new skin ulceration
or blistering) [22].
•
Surgical consultation should be obtained whenever there is concern for a severe extravasation injury [11, 22]; this
can be suspected if the patient develops severe pain, progressive swelling or pain, decreased capillary refill,
change in sensation, worsening active or passive range of motion in the elbow, wrist, or fingers, or skin ulceration
or blistering [17]; reliance on an extravasation volume threshold to trigger surgical consultation is not
recommended [11, 13, 18].
Power-injection through central venous catheters and peripherally inserted central catheters (PICCs)
•
Contrast material can only be power-injected into central venous catheters [38] or PICCs [39] if these catheters
have been certified for such use, with the flow-rate limit provided. All manufacturer recommendations should be
followed.
Gadolinium-based contrast media
•
Extravasation injuries after injection of gadolinium-based contrast media are much less common than those seen after
injection of iodinated contrast material [20], likely due, in part, to less toxicity [21] and the low volumes of gadolinium-
based contrast media that are injected.
EXTRAVASATION OF CONTRAST MEDIA 18
Summary Questions and Answers
(Study Quality = SQ)
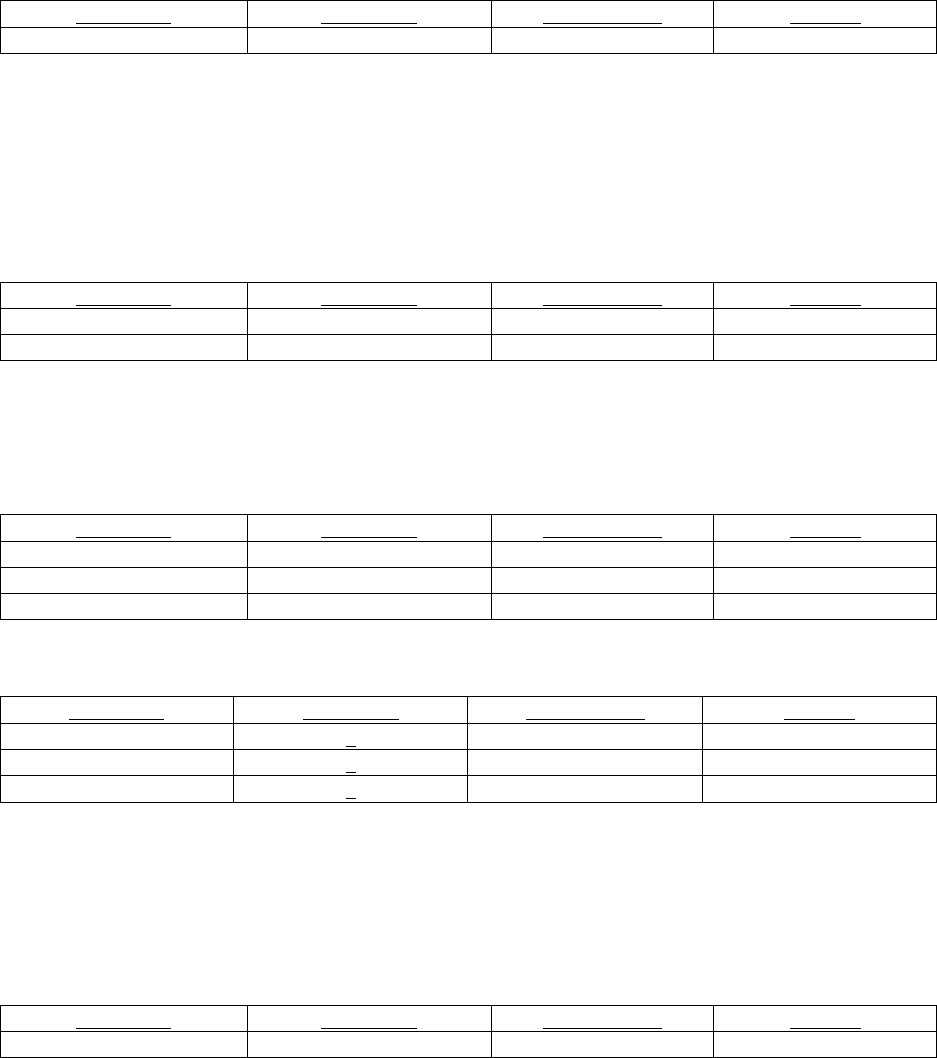
Question 1. What actions can be performed to minimize the likelihood of an extravasation?
Recommendation 1: Extravasation risk is minimized by 1) using angiocatheters over butterflies, 2) performing meticulous
intravenous insertion technique (confirming intravenous location by aspirating blood through an inserted catheter and flushing the
inserted catheter with a test injection), 3) and carefully securing an inserted catheter.
Strength of evidence: Limited 1
Reference #
Original SQ
Reassessed SQ
Final SQ
41
2
-
2
Question 2: What risk factors should clinicians take into consideration in determining the likelihood of a contrast
extravasation?
Recommendation 1: Clinicians should consider patient related factors such as history of altered circulation in the injected
extremity, prior radiation to the injected extremity, or uncommunicative patients.
Strength of evidence: Limited 1
Reference #
Original SQ
Reassessed SQ
Final SQ
1
2
-
2
32
4
-
4
Recommendation 2: Clinicians should consider contrast and injection parameters such as viscosity of contrast material and
location of injection other than non-antecubital fossa regions (such as hand, foot, and ankle more at risk)
Strength of evidence: Viscosity = Strong 3
Reference #
Original SQ
Reassessed SQ
Final SQ
6
2
-
2
8
2
-
2
35
2
-
2
Strength of evidence: Location of injection = Strong 3
Reference #
Original SQ
Reassessed SQ
Final SQ
1
2
-
2
20
4
-
4
36
2
-
2
Question 3: How should clinicians evaluate patients for potential contrast extravasation?
Recommendation 1: Clinicians should do a physical exam of the affected extremity to evaluate for tenderness, swelling,
erythema, paresthesia, active and passive range of finger motion, and perfusion.
Strength of evidence: Limited 3
Reference #
Original SQ
Reassessed SQ
Final SQ
22
4
-
4
EXTRAVASATION OF CONTRAST MEDIA 19
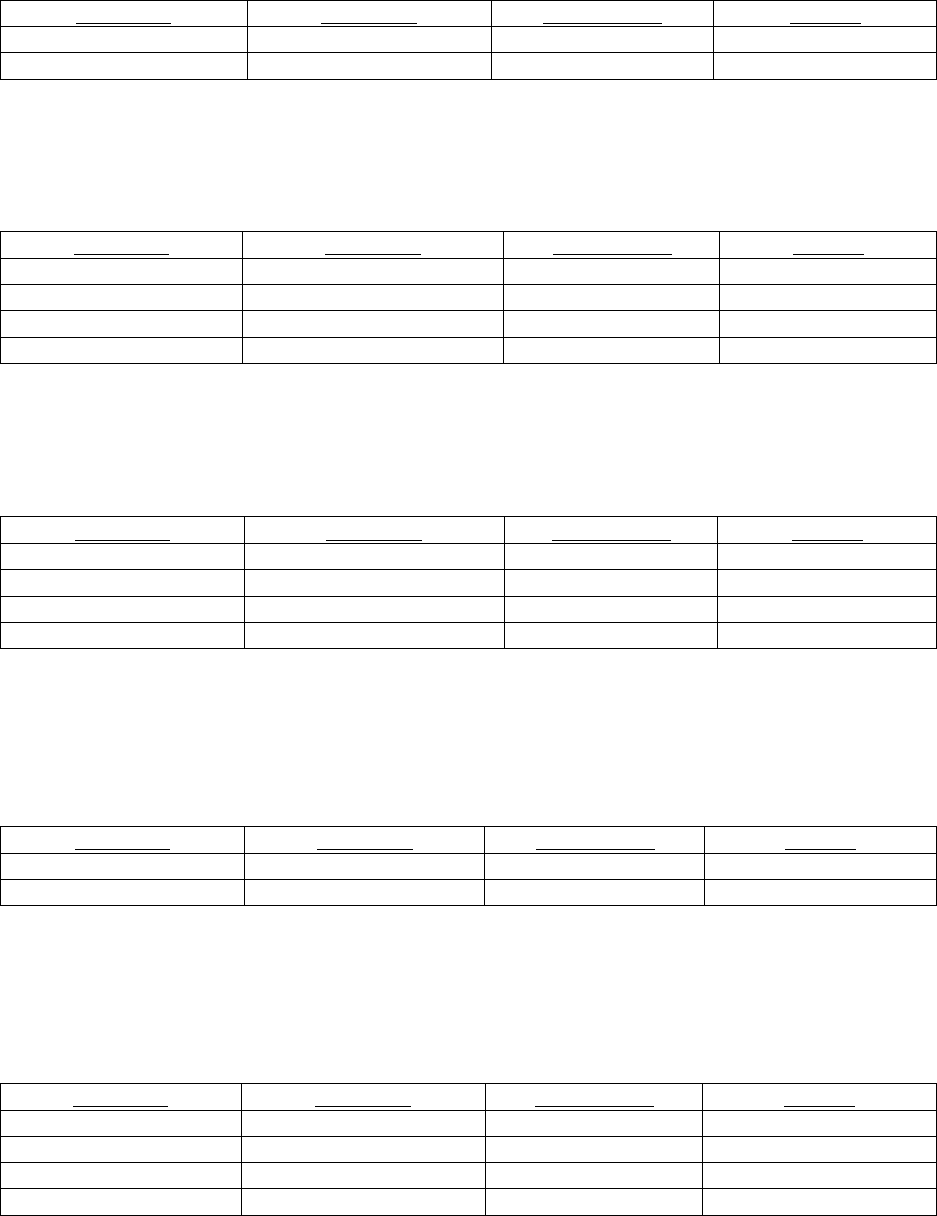
Question 4: How should contrast extravasations be treated?
Recommendation 1: Elevation of the affected extremity above the heart.
Strength of evidence: Limited 3
Reference #
Original SQ
Reassessed SQ
Final SQ
22
4
-
4
24
4
-
4
Recommendation 2: Cold compresses or ice packs should initially be applied to the extravasation site (rather than warm
compresses)
Strength of evidence: Limited 1
Reference #
Original SQ
Reassessed SQ
Final SQ
22
4
-
4
23
Inadequate
-
Inadequate
24
4
-
4
25
1
-
1
Recommendation 3: Routine use of hyaluronidase or corticosteroid injections or aspiration of the affected limb is not
recommended.
Strength of evidence: Limited 1
Reference #
Original SQ
Reassessed SQ
Final SQ
15
4
4
4
22
4
-
4
28
4
-
4
29
2
-
2
Recommendation 4: All discharged outpatients should be given clear instructions concerning where and when to seek additional
medical care (including for worsening pain, development of paresthesia, diminished range of motion, and new skin ulceration o r
blistering) as severe extravasation could develop several hours later.
Strength of evidence: Limited 3
Reference #
Original SQ
Reassessed SQ
Final SQ
11
4
3
3
22
4
-
4
Question 5: When should surgical consultation be placed?
Recommendation 1: Surgical consultation should not be routinely requested based on volume alone.
Strength of evidence: Strong 3
Reference #
Original SQ
Reassessed SQ
Final SQ
11
4
3
3
13
4
4
4
18
1
-
1
14
2
-
2
EXTRAVASATION OF CONTRAST MEDIA 20
Recommendation 2: Surgical consultation should be requested whenever there is concern for a severe extravasation injury; this
can be suspected if the patient develops severe pain, progressive swelling or pain, decreased capillary refill, change in sensation,
worsening active or passive range of motion in the elbow, wrist, or fingers, or skin ulceration or blistering.
Strength of evidence: Limited 3
Reference #
Original SQ
Reassessed SQ
Final SQ
11
4
3
3
22
4
-
4
Question 6: Can automated (power) injectors be utilized for injections in central venous or PICC lines?
Recommendation 1: Use central venous catheter to power inject contrast or PICCs if the catheters have been certified for such
use, with the flow-rate limit provided. All manufacturer recommendations should be followed.
Strength of evidence: Limited 1
Reference #
Original SQ
Reassessed SQ
Final SQ
38
2
-
2
39
4
-
4
Question 7: What is the extravasation risk from injection of gadolinium-based contrast media?
Recommendation 1: Extravasation injuries are extremely unlikely during gadolinium-based contrast media injection, likely due
to lower toxicity than iodinated contrast agents and lower total volumes of injected contrast media.
Strength of evidence: Limited 3
Reference #
Original SQ
Reassessed SQ
Final SQ
20
4
-
4
21
4
-
4
EXTRAVASATION OF CONTRAST MEDIA 21
EXTRAVASATION OF CONTRAST MEDIA
Frequency
The reported incidence of intravenous (IV) contrast media extravasation in adults and children related to power injection for
CT has ranged from 0.1% to 1.2% [41-49] (1/1,000 patients to 1/83 patients). Extravasation can also occur during hand
injections. Extravasations may occur at both low and high flow rates [50]. Extravasation occurring with dynamic bolus CT may
involve large volumes of contrast media [51].
Initial Signs and Symptoms
Most extravasations are limited to the immediately adjacent soft tissues (typically the skin and subcutaneous tissues).
Although most patients complain of initial swelling or tightness, and/or stinging or burning pain at the site of extravasation,
some experience little or no discomfort [51,52]. On physical examination, the extravasation site may be edematous,
erythematous, and tender [51].
Extravasation of Iodinated Contrast Material
In most patients, initial swelling and tenderness resolves within hours to days after the extravasation. The vast majority of
patients in whom extravasations occur recover without clinically important sequelae [46,47,51,53,54]. However, in some
patients, extravasated iodinated contrast media can result in injury to surrounding tissues, particularly the skin, producing an
acute local inflammatory response that peaks at 24 to 48 hours [55]. Most of the time there are no lasting complications. Only
rarely will a low-osmolality contrast media (LOCM) extravasation injury proceed to a severe adverse event [51]. Acute tissue
injury resulting from extravasation of iodinated contrast media is probably related at least in part to its hyperosmolality [56,57].
Several large series have illustrated the infrequency of severe injuries after LOCM extravasation. In one single institutional
study, all 321 extravasation injuries were mild [48]. In another single institutional study [51], only one of 442 adult LOCM
extravasations resulted in a severe injury (a compartment syndrome). Three other patients developed blisters or ulcerations that
were successfully treated locally. In a third study, utilizing a practice quality improvement database established by the American
College of Radiology [47] only six of 1,085 reported extravasations resulted in severe injuries, and only one patient required
surgical intervention.
The most commonly reported severe injury after LOCM extravasation is compartment syndrome [51]. Compartment syndrome
results from mechanical compression and is probably more likely to occur after extravasation of larger volumes of contrast
media; however, it also has been observed after extravasation of small volumes, especially when these occur in less capacious
areas (such as over the ventral or dorsal surfaces of the wrist) [51]. Compartment syndrome may develop soon after an
extravasation [51] or result from swelling that sometimes occurs hours after the extravasation [58].
Less commonly encountered severe injuries include skin ulceration and tissue necrosis [59]. These can occur within hours or
days of the extravasation event.
Extravasation of Gadolinium Based Contrast Media
Extravasation of gadolinium-based contrast media is less common (i.e., approximately one-sixth as often) than iodinated
contrast media [60]. The difference is likely the due to much lower volumes of administered gadolinium-based contrast media
for most clinical indications. Additionally, on a cc to cc basis, gadolinium-based contrast media may also have less toxicity
than iodinated contrast agents [61].
Evaluation of Patients in Whom Extravasations Occur
A responsible health care provider should be summoned to examine any patient in whom an extravasation of contrast material
has occurred. The patient should be asked about symptoms of pain and paresthesias. A brief examination should be performed
and should include assessment of extremity tenderness, swelling, erythema, paresthesia, active and passive range of motion of
the fingers, and perfusion [62].
Treatment
Studies evaluating potential treatments of extravasation injuries are generally of low quality [63]. Given the absence of any
studies demonstrating definite efficacy of any specific treatment regimen, the optimum treatment of extravasation events has
EXTRAVASATION OF CONTRAST MEDIA 22
not been determined. Our limited guidance is to suggest treatments that have a low risk of harm, but that might have some
efficacy.
Extravasations that resolve rapidly probably need no treatment other than brief observation and discharge instructions (see
section entitled Discharging Patients in Whom Extravasations Occur, below). More symptomatic extravasations may be treated
with extremity elevation above the level of the heart (to decrease capillary hydrostatic pressure and thereby promote resorption
of extravasated fluid [22, 24]) and either warm or cold compresses that an outpatient may continue intermittently at home until
symptoms resolve, along with discharge instructions, or may be continued on the wards for inpatients. Controlled studies
demonstrating the efficacy of these interventions are lacking, however [25]. There is no clear evidence favoring the use of
warm over cold compresses or vice versa [64]. Some surgeons empirically recommend initial use of cold compresses to promote
vasoconstriction and diminish inflowing blood and swelling [62,65].
Severe cases in which there is concern for neurologic or vascular compromise or skin ulceration and necrosis require surgical
consultation to assess the need for operative management (see next section).
There is no consistent evidence that the effects of an extravasation can be mitigated by trying to aspirate the extravasated
contrast medium through an inserted needle or angiocatheter [62,66]. When such attempts are made, usually little or no
extravasated contrast material can be successfully aspirated. Therefore, aspiration is not recommended.
Some have suggested that the extravasation site can be treated by performing multiple punctures around the extravasation site
and then manually squeezing the site [67,68]; however, the effectiveness of this approach has not been validated and it is not
recommended.
Topical application of silver sulfadiazine ointment and steroid cream three to four times daily has been recommended by some
as an approach to soothe irritated skin, reduce inflammation, and to prevent infection should any blistering occur, although the
efficacy of this treatment is unknown [52].
There is no consistent evidence that local injection of potentially therapeutic agents, such as corticosteroids or hyalurinidase is
beneficial [55,69,70]. Hyaluronidase has been used in the management of extravasation events for medications unrelated to
contrast media, and there are a few case reports in which it was attempted following a contrast material extravasation event [71-
73]. However, no adequate studies confirm efficacy of hyaluronidase after contrast media extravasation [68]. Therefore, use of
hyaluronidase for the management of contrast material extravasation is not recommended [62].
Surgical Consultation
Urgent surgical consultation should be obtained whenever there is concern for a severe extravasation injury [11,22]. Although
consultation can prolong length of stay (eg, by 2.5 hours in one ED population [14]), it should be obtained for any patient in
whom one or more of the following extravasation-related signs or symptoms develops: severe pain; progressive swelling or
pain; altered tissue perfusion as evidenced by decreased capillary refill; change in sensation in the affected limb; worsenin g
passive or active range of motion; and skin ulceration or blistering [17].
Reliance on an extravasation volume threshold (such as estimated volumes exceeding 100 or 150 mL) to indicate the need for
surgical consultation has been recommended by some [51,53]. Although a severe injury is probably more likely when larger
volumes are involved, most patients with large volume extravasation do not develop severe complications, even when distal to
the elbow [53]. Because of this, surgical consultation should be based on signs and symptoms rather than an absolute volume
threshold. If the patient is asymptomatic or has only mild symptoms, appropriate evaluation and clinical follow-ups are usually
sufficient.
Discharging Patients in Whom Extravasations Occur
Outpatients who have suffered contrast media extravasation should be released from the radiology department only after an
initial period of observation, provided the radiologist is satisfied that any signs and symptoms that were present initially have
improved or that new symptoms have not developed during the observation period. Clear instructions should be given to the
patient to seek additional medical care for severe pain, progressive pain, numbness or tingling, diminished range of motion
(active or passive), skin ulceration, or other neurologic or circulatory symptoms [62]. This is because initial symptoms of a
serious compartment syndrome may be absent or relatively mild (such as limited to the development of focal paresthesia) [58].
EXTRAVASATION OF CONTRAST MEDIA 23
Other Considerations
Patients at Increased Risk for Extravasations
Extravasations are more common in patients who cannot communicate effectively (e.g., the elderly, infants and children, and
patients with altered consciousness), severely ill or debilitated patients, and patients with abnormal circulation in the limb to be
injected [74]. Patients with altered circulation include those with atherosclerotic peripheral vascular disease, diabetic vascular
disease, Raynaud’s disease, venous thrombosis or insufficiency, or prior radiation therapy or extensive surgery (e.g., axilla ry
lymph node dissection or saphenous vein graft harvesting) in the limb to be injected. Women may have a mild increased risk of
extravasation [63]. Some of these conditions are systemic and cannot be avoided by choosing a different injection site.
Certain intravenous access sites (e.g., hand, wrist, foot, and ankle) are more likely to result in extravasation and should be
avoided, when possible [41]. However, use of these alternate injection sites may be necessary due to lack of availability of the
more traditional locations.
In some studies, extravasations were more common in patients injected through small-bore (22 gauge) catheters compared to
larger-bore catheters (2.2% versus 1.0-1.1%) [41].
While some reviews have found that injection at higher flow rates likely increases the risk of extravasation [48], in others, no
such difference has been detected [41,75]. In fact, it has been shown that flow rates of up to 3 and 5 mL/sec can be safely achieved
through 22 gauge and 20 gauge intravenous catheters, respectively, in the vast majority of patients, provided that there is no
increased resistance or pain during a rapid test injection [75].
Patients injected with more viscous contrast material may be more likely to have extravasations than are patients injected with
less viscous contrast material [46,48,76]. This effect may be mitigated for viscous media by extrinsic warming to human body
temperature prior to injection [76].
The risk of extravasation also appears to be increased in patients in whom deep brachial intravenous access is achieved under
ultrasound guidance, a practice used more often in Emergency Departments for patients in whom IV access is otherwise difficult
[77]. In one series, contrast media extravasation occurred during CT in 0.3% of all patients, but in 3.6% of patients who were
injected through ultrasound-guided peripheral intravenous catheters [78]. That difference is likely in part due to selection bias
(patients requiring ultrasound-guided access likely have more difficult venous access).
Extravasation Risk of Injection through Indwelling Central Venous Catheters
All central venous catheter port sites can be utilized for venous access if gentle hand injections are performed; however, only
certain central venous catheter port sites are certified for use with mechanical injectors. Prior to using a central venous line
for power injection, it is important to ensure that the port site is certified as power-injectable, and the flow limit should be
noted. If this process is followed, it can be safe. In one report of 142 injections through certified power port sites (11 at high
rates up to 5 mL/second), there were no extravasations [79].
Mechanical injections can also be performed through some pressure-injectable peripherally inserted central catheters
(PICCs). Manufacturer recommendations should be followed. There have been isolated reports of PICC tip migrations
following pressure injections through these catheters [80].
Patients at Increased Risk for a Severe Extravasation Injury Once an Extravasation Occurs
A severe extravasation injury may be more likely to result from an extravasation in certain patients, such as those with arterial
insufficiency or compromised venous or lymphatic drainage in the affected extremity. In addition, extravasations involving
larger volumes of contrast media and those occurring in the dorsum of the hand, foot, or ankle are more likely to result in
severe injury [22, 32].
Preventing Extravasation Injuries
Methods for reducing the risk of extravasation include: meticulous intravenous line insertion technique, using angiocatheters
instead of butterfly catheters, confirming position by aspirating blood (although failure to aspirate blood does not exclude the
possibility of proper catheter location), flushing an inserted catheter with a test injection of saline to ensure proper flow into
the accessed vein, and carefully securing the inserted catheter [81].
Low-risk intravenous line insertion sites are preferred when feasible. If not feasible, higher risk sites may be considered
depending on the risks and benefits of administrating contrast media for the examination indication. Use of a preliminary
EXTRAVASATION OF CONTRAST MEDIA 24
saline flush to assess injection pressure prior to contrast media administration has been advocated by a few investigators [82];
however, this has not been adopted by most institutions. Some have recommended use of a hand-held alarm, which the patient
can press should any new symptoms develop [52]. Interestingly, the practice quality improvement project created by the ACR
to assist practices in identifying improvements that could be made to reduce the frequency of extravasations did not find that
any significant improvement could be achieved, even when risk factors for extravasations were identified and attempts were
made to reduce the extravasation risk in advance [47].
Documentation
All extravasation events and their treatment should be documented in the medical record. In addition, the referring provider
should be notified following any symptomatic extravasation.
Revision History
23 January 2018 (Minor Revision)
October 2021 (Evidence Based Update)
References
1. Wienbeck S, Fischbach R, Kloska SP, et al. Prospective study of access site complications of automated contrast injection with peripheral venous
access in MDCT. AJR Am J Roentgenol 2010;195:825-9.
2. Sistrom CL, Gay SB, Peffley L. Extravasation of iopamidol and iohexol during contrast-enhanced CT: report of 28 cases. Radiology 1991;180:707-
10.
3. Sinan T, Al-Khawari H, Chishti FA, Al Saeed OM, Sheikh M. Contrast media extravasation: manual versus power injector. Med Princ Pract
2005;14:107-10.
4. Jacobs JE, Birnbaum BA, Langlotz CP. Contrast media reactions and extravasation: relationship to intravenous injection rates. Radiology
1998;209:411-6.
5. Federle MP, Chang PJ, Confer S, Ozgun B. Frequency and effects of extravasation of ionic and nonionic CT contrast media durin g rapid bolus
injection. Radiology 1998;206:637-40.
6. Barrera CA, White AM, Shepherd AM, et al. Contrast Extravasation using Power Injectors for Contrast -Enhanced Computed Tomography in
Children: Frequency and Injury Severity. Academic radiology 2019;26:1668-74.
7. Dykes TM, Bhargavan-Chatfield M, Dyer RB. Intravenous contrast extravasation during CT: a national data registry and practice quality
improvement initiative. Journal of the American College of Radiology : JACR 2015;12:183-91.
8. Hwang EJ, Shin CI, Choi YH, Park CM. Frequency, outcome, and risk factors of contrast media extravasation in 142,651 intraven ous contrast-
enhanced CT scans. European radiology 2018;28:5368-75.
9. Kingston RJ, Young N, Sindhusake DP, Truong M. Study of patients with intravenous contrast extravasation on CT studies, with radiology staff
and ward staff cannulations. Journal of medical imaging and radiation oncology 2012;56:163-7.
10. Cohan RH, Bullard MA, Ellis JH, et al. Local reactions after injection of iodinated contrast material: detection, management, and outcome.
Academic radiology 1997;4:711-8.
11. Wang CL, Cohan RH, Ellis JH, Adusumilli S, Dunnick NR. Frequency, management, and outcome of extravasation of nonionic iodinated contrast
medium in 69,657 intravenous injections. Radiology 2007;243:80-7.
12. Ko CH, Tay SY, Chang HC, Chan WP. Large-volume iodinated contrast medium extravasation: low frequency and good outcome after
conservative management in a single-centre cohort of more than 67,000 patients. European radiology 2018;28:5376-83.
13. Sbitany H, Koltz PF, Mays C, Girotto JA, Langstein HN. CT contrast extravasation in the upper extremity: strategies for management. International
journal of surgery (London, England) 2010;8:384-6.
14. Sonis JD, Gottumukkala RV, Glover M, et al. Implications of iodinated contrast media extravasation in the emergency departmen t. American
Journal of Emergency Medicine 2018;36:29T 296.
15. McAlister WH, Palmer K. The histologic effects of four commonly used media for excretory urography and an attempt to modify t he responses.
Radiology 1971;99:511-6.
16. McAlister WH, Kissane JM. Comparison of soft tissue effects of conventional ionic, low osmolar ionic and nonionic iodine containing contrast
material in experimental animals. Pediatric radiology 1990;20:170-4.
17. Cohan RH, Leder RA, Bolick D, et al. Extravascular extravasation of radiographic contrast media. Effects of conventional and low-osmolar agents
in the rat thigh. Invest Radiol 1990;25:504-10.
18. Memolo M, Dyer R, Zagoria RJ. Extravasation injury with nonionic contrast material. AJR Am J Roentgenol 1993;160:203-4.
19. Pond GD, Dorr RT, McAleese KA. Skin ulceration from extravasation of low-osmolality contrast medium: a complication of automation. AJR Am
J Roentgenol 1992;158:915-6.
20. Heshmatzadeh Behzadi A, Farooq Z, Newhouse JH, Prince MR. MRI and CT contrast media extravasation: A systematic review. Medic ine
2018;97:e0055.
21. Cohan RH, Leder RA, Herzberg AJ, et al. Extravascular toxicity of two magnetic resonance contrast agents. Preliminary experience in the rat.
Invest Radiol 1991;26:224-26.
22. Cohan RH, Ellis JH, Garner WL. Extravasation of radiographic contrast material: recognition, prevention, and treatment. Radiology 1996;200:593-
604.
23. Ding S, Meystre NR, Campeanu C, Gullo G. Contrast media extravasations in patients undergoing computerized tomography scannin g: a
systematic review and meta-analysis of risk factors and interventions. JBI database of systematic reviews and implementation reports 2018;16:87-
116.
24. Bellin MF, Jakobsen JA, Tomassin I, et al. Contrast medium extravasation injury: guidelines for prevention and management. Eu ropean radiology
2002;12:2807-12.
EXTRAVASATION OF CONTRAST MEDIA 25
25. Hastings-Tolsma MT, Yucha CB, Tompkins J, Robson L, Szeverenyi N. Effect of warm and cold applications on the resolution of i.v. infiltrations.
Research in nursing & health 1993;16:171-8.
26. Sum W, Ridley LJ. Recognition and management of contrast media extravasation. Australas Radiol 2006;50:549-52.
27. Kim SM, Cook KH, Lee IJ, Park DH, Park MC. Computed tomography contrast media extravasation: treatment algorithm and immediat e treatment
by squeezing with multiple slit incisions. International wound journal 2017;14:430-34.
28. Mandlik V, Prantl L, Schreyer AG. Contrast Media Extravasation in CT and MRI - A Literature Review and Strategies for Therapy. RoFo :
Fortschritte auf dem Gebiete der Rontgenstrahlen und der Nuklearmedizin 2019;191:25-32.
29. Park KS, Kim SH, Park JH, Han MC, Kim DY, Kim SJ. Methods for mitigating soft-tissue injury after subcutaneous injection of water soluble
contrast media. Invest Radiol 1993;28:332-4.
30. Rowlett J. Extravasation of contrast media managed with recombinant human hyaluronidase. The American journal of emergency medicine
2012;30:2102.e1-3.
31. Hanrahan K. Hyaluronidase for treatment of intravenous extravasations: implementation of an evidence-based guideline in a pediatric population.
Journal for specialists in pediatric nursing : JSPN 2013;18:253-62.
32. Upton J, Mulliken JB, Murray JE. Major intravenous extravasation injuries. American journal of surgery 1979;137:497-506.
33. Lewis GB, Hecker JF. Radiological examination of failure of intravenous infusions. Br J Surg 1991;78:500-1.
34. Schwab SA, Uder M, Anders K, Heinrich MC, Kuefner MA. Peripheral intravenous power injection of iodinated contrast media through 22G and
20G cannulas: can high flow rates be achieved safely? A clinical feasibility study. RoFo : Fortschritte auf dem Geb iete der Rontgenstrahlen und der
Nuklearmedizin 2009;181:355-61.
35. Davenport MS, Wang CL, Bashir MR, Neville AM, Paulson EK. Rate of contrast material extravasations and allergic -like reactions: effect of
extrinsic warming of low-osmolality iodinated CT contrast material to 37 degrees C. Radiology 2012;262:475-84.
36. Hardie AD, Kereshi B. Incidence of intravenous contrast extravasation: increased risk for patients with deep brachial catheter placement from the
emergency department. Emergency radiology 2014;21:235-8.
37. Rupp JD, Ferre RM, Boyd JS, et al. Extravasation Risk Using Ultrasound-guided Peripheral Intravenous Catheters for Computed Tomography
Contrast Administration. Academic emergency medicine : official journal of the Society for Academic Emergency Medicin e 2016;23:918-21.
38. Alexander MD, Morrison HL. Power-injectable ports: safety during placement, therapeutic use, and contrast administration during computed
tomography procedures. The journal of vascular access 2012;13:432-7.
39. Craigie M, Meehan L, Harper J. Tip Migration Post-Contrast Pressure Injection Through Pressure-Injectable Peripherally Inserted Central Catheters
Causing Vascular Injury: A Report of 3 Cases. Cardiovascular and interventional radiology 2018;41:509-12.
40. Kadom N, Hashim HD, Olsen C, Cefaratti M, Bulas D, Shalaby-Rana E. Nursing role model for computed tomography contrast injection decreases
extravasation rates. Journal of pediatric nursing 2012;27:113-8.
41. Abe S, Mizuno N, Tani S, et al. Effectiveness of the new injection program 'saline test injection mode' for use power injector in pediatric contrast
CT. Australasian physical & engineering sciences in medicine 2013;36:347-54.
ALLERGIC-LIKE AND PHYSIOLOGIC REACTIONS TO INTRAVASCULAR IODINATED CONTRAST MEDIA 26
ALLERGIC-LIKE AND PHYSIOLOGIC REACTIONS TO
INTRAVASCULAR IODINATED CONTRAST MEDIA
The frequency of allergic-like and physiologic adverse events related to the intravascular administration of iodinated contrast
media (ICM) is low and has decreased considerably with changes in usage from ionic high- osmolality contrast media
(HOCM) to nonionic low-osmolality contrast media (LOCM) [1-11]. The majority of adverse side effects to LOCM are mild
non-life-threatening events that usually require only observation, reassurance, and/or supportive measures [3,12,13]. Severe
and potentially life-threatening adverse events continue to occur rarely and unpredictably. Nearly all life-threatening contrast
reactions occur within the first 20 minutes after contrast medium injection.
All personnel who inject intravascular contrast media should be prepared to: 1) recognize the variety of adverse events that
may occur following ICM administration and 2) institute appropriate measures to manage the reaction. These measures
include notifying the supervising radiologist (or his/her designee), monitoring the patient, administering certain medications,
and/or calling for additional assistance (emergency service providers, “code team”, etc.).
Acute Adverse Events
Classification of Acute Adverse Events
Acute adverse events can be categorized as either allergic-like or physiologic and organized into three general categories of
severity (mild, moderate, or severe). A suggested classification system (which can be utilized for both ICM and gadolinium-
based contrast media [GBCM]), stratifying adverse events by severity and type, is presented in Table 1.
A standardized classification system is important to minimize variation between published reports. It is of particular
importance to avoid contaminating the reported incidence of allergic-like reactions with that of physiologic reactions,
because the management of patients experiencing these reaction types is different (e.g., patients who experience allergic-like
reactions may require future premedication prior to ICM- enhanced studies, while patients who experience physiologic
reactions would not).
Allergic-Like Reactions
Allergic-like reactions to ICM manifest similarly to true allergic reactions seen with other drugs and allergens, but because
an antigen-antibody response cannot be always identified, allergic-like contrast reactions are classified as “anaphylactoid”,
“allergic-like”, or “idiosyncratic” [2,3,12,13]. Treatment of an allergic-like contrast reaction is identical to that of an
equivalent allergic reaction. Allergic-like contrast reactions are likely independent of dose and concentration above a certain
unknown threshold [3].
The pathogenesis of most allergic-like reactions is unclear. There are multiple possible mechanisms that result in activation
of immunologic effectors [14]. It is believed that some allergic-like contrast reactions may involve activation, deactivation,
or inhibition of a variety of vasoactive substances or mediators (such as histamine, complement, and the kinin system) [3,12-
15]. ICM are known to directly cause histamine release from basophils and mast cells [9]. Histamine release must have
occurred when patients develop urticaria, but the precise cause and pathway of histamine release are not known [3,12,13].
Skin and intradermal testing are positive in a minority of individuals, indicating that an allergic IgE-mediated etiology may
be responsible for some reactions [16], but this is the minority of cases.
Additives or contaminants, such as calcium-chelating substances or substances eluted from rubber stoppers in bottles or
syringes, have been suggested as contributory in some allergic-like contrast reactions [12,13].
Physiologic Reactions
Physiologic reactions to ICM likely relate to specific molecular attributes that lead to direct chemotoxicity [3,12,13],
osmotoxicity (adverse effects due to hyperosmolality) [14], or molecular binding to certain activators [9]. Physiologic
reactions are frequently dose and concentration dependent [3].
Cardiac arrhythmias, depressed myocardial contractility, cardiogenic pulmonary edema, and seizures are very rare,
potentially serious physiologic reactions to ICM [3,9,12,13]. These phenomena are likely related to either contrast media -
related hyperosmolality and/or calcium binding leading to functional hypocalcemia [3,9,12,13]. Cardiac adverse events are
much more common during angiocardiography than intravenous ICM administration.

ALLERGIC-LIKE AND PHYSIOLOGIC REACTIONS TO INTRAVASCULAR IODINATED CONTRAST MEDIA 27
Cardiovascular effects are more frequent and significant in patients with underlying cardiac disease. For example, patients
with left heart failure are less able to compensate for the osmotic load and minor negative chronotropic effects of ICM. As
a result, there is an increased risk of developing acute pulmonary edema. Noncardiogenic pulmonary edema can also very
rarely occur following intravascular ICM administration [16], although it is unclear whether this represents a physiologic or
allergic-like reaction.
Vasovagal reactions are relatively common and characterized by hypotension with bradycardia. While the exact pathogenesis
is unknown, this particular response is thought to be the result of increased vagal tone arising from the central nervous
system. The effects of increased vagal tone include depressed sinoatrial and atrioventricular nodal activity, inhibition of
atrioventricular conduction, and peripheral vasodilatation [3]. Vasovagal reactions may be related to anxiety and can occur
while informed consent is being obtained, during placement of a needle or catheter for contrast medium injection, or during
intravascular administration of contrast media. Such reactions commonly present with a feeling of apprehension and
accompanying diaphoresis [3].
While most vagal reactions are mild and self-limited, close patient observation is recommended until symptoms resolve
fully. Severe hypotension may very rarely cause loss of consciousness, cardiovascular collapse, angina, or seizures [3].
Patient anxiety may also contribute to or exacerbate nonvagal adverse events. Similar to allergic-like reactions, some additives
and contaminants have been associated with physiologic reactions [12,13].
For a discussion of renal failure, please see the separate chapter on Contrast-Induced Nephrotoxicity.
Frequency of Acute Adverse Events
The frequency of acute adverse events after the administration of intravascular ICM is difficult to determine with precision
because similar signs and symptoms may arise from concomitant medical conditions, medications, anxiety, etc.
Underreporting and variation in the classification of acute adverse reactions have affected the reported incidence of these
events.
Historically, acute adverse events occurred in 5% to 15% of all patients who received HOCM. Many patients receiving
intravascular HOCM experienced physiologic disturbances (e.g., generalized warmth, nausea, or emesis), and this was often
documented as a contrast reaction. HOCM are now rarely or never used for intravascular purposes because of their greater
adverse event profile compared to LOCM.
LOCM are associated with a very low incidence of acute adverse events, and the bulk of these are not life- threatening.
Cochran et al [17] reported an overall acute adverse reaction rate (allergic-like + physiologic) of 0.2% for nonionic LOCM
administered at a single institution. A slightly higher overall frequency of 0.7% (allergic-like + physiologic) was reported
from another institution upon review of 29,508 patients given iopromide over a 2-year period [18]. Wang et al [19] reported
an overall acute allergic-like reaction frequency of 0.6% in 84,928 adult patients who received iohexol, iopromide, or
iodixanol.
A single institutional study of pediatric patients receiving intravenous LOCM by Dillman et al [20] demonstrated a frequency
of acute allergic-like reactions of 0.18%. Another single institutional study in children by Callahan et al [21] demonstrated
an overall acute adverse reaction rate of 0.46% (allergic-like + physiologic).
Serious acute reactions to IV LOCM are rare, with an historical rate of approximately four in 10,000 (0.04%) [6].
The mortality incidence related to intravascular ICM is unknown. In a large Japanese study by Katayama et al [6], no fatal
reactions were attributed to LOCM despite greater than 170,000 injections. The conservative estimate of 1 fatality per
170,000 contrast media administrations is thus often quoted. Fatal reactions to LOCM have been reported [4,17,18,22,23].
A meta-analysis performed by Caro et al [4] documented a fatality rate of 0.9 per 100,000 injections of LOCM. A review of
U.S. FDA and drug manufacturer data from 1990 to 1994 demonstrated 2.1 fatalities per 1 million contrast-enhanced studies
using LOCM [7].
Common Risk Factors for Acute Contrast Reactions
Although it is clear that certain patients are at increased risk of experiencing an adverse event to intravascular ICM, contrast
reactions remain sporadic and unpredictable.

ALLERGIC-LIKE AND PHYSIOLOGIC REACTIONS TO INTRAVASCULAR IODINATED CONTRAST MEDIA 28
A prior allergic-like reaction to ICM is the most substantial risk factor for a recurrent allergic-like adverse event
[1,2,6,18,24]. Such a history is not an absolute predictor, and the incidence of recurrent allergic-like reactions in high-risk
nonpremedicated patients is unknown. It is estimated to range from 10 to 35% [6,25,26]. The estimated risk in high-risk
premedicated patients is estimated to be approximately 10% [26,27]. Atopic individuals (particularly those with multiple
severe allergies) and asthmatics are also at increased risk for allergic-like contrast reactions, although probably not to as
great an extent [3,6,9,12,13,24,25,28]. Those with a history of prior allergic-like reaction to GBCM are at no greater risk for
allergic-like reaction to ICM than other patients with a similar number of allergies and other risk factors (e.g., asthma). A
prospective study by Kopp et al [24] of over 74,000 patients who received iopromide demonstrated that certain age and
gender combinations (e.g., young females) may have a higher incidence of allergic-like reactions compared to the general
population. A retrospective case-control study by Lang et al [28] showed that individuals with asthma and those receiving
beta-adrenergic blocker therapy may be at increased risk for moderate and severe reactions; however, this study did not
match patients based on underlying diseases and it is possible that beta- blocker therapy merely indicated those patients with
more comorbid conditions.
Pre-existing medical conditions may increase the risk of certain adverse events. For example, bronchospasm is a common
adverse event among patients with a history of asthma. Hemodynamic changes are more common in patients with
significant cardiovascular disease, such as aortic stenosis or severe congestive heart failure.
The effects of dose, route (intravenous vs. intra-arterial vs. other), and rate of delivery of contrast media on the incidence of
adverse events are not entirely clear. Studies have shown that a “test injection” does not decrease the incidence of severe
allergic-like reactions [29,30], and may actually increase it. Non-reaction to a “test injection” does not indicate that an
allergic-like reaction will not occur with a standard injection [25]. Test injections are not recommended for predicting which
patients will react to ICM.
Patients with Myasthenia Gravis
Myasthenia gravis has historically been considered a relative contraindication to intravascular iodinated contrast material
exposure based on experimental and largely anecdotal clinical data with respect to HOCM. Due to a lack of clear evidence
showing adverse effects for LOCM in this setting, only a few contrast material manufacturers continue to suggest precaution
in patients with myasthenia gravis.
However, Somashekar et al [31] in 2013 studied 267 patients with clinically confirmed myasthenia gravis who underwent
CT (112 with LOCM (CE-CT), 155 without LOCM (NC-CT)), and showed a significantly greater fraction of disease-related
symptom exacerbations within 24 hours in the CE-CT group (6.3% [7/112] for CE-CT vs. 0.6% [1/155] for NC-CT, p =
0.01). These findings suggest that intravascular LOCM may be relatively contraindicated in patients with myasthenia gravis.
This is the first evidence of such a relationship in the medical literature, and confirmatory studies will be needed before a
more definitive recommendation can be made.
Other Risk Factors
Drug package inserts suggest precautions are necessary to avoid adverse events in patients with known or suspected
pheochromocytoma, thyrotoxicosis, dysproteinemias, or sickle-cell disease. There are scant data, however, to support the
need for specific precautions in these patients when LOCM is used (See the Chapter on Patient Selection and Preparation
Strategies). For example, a small retrospective study by Bessell- Browne and O’Malley [32] demonstrated no adverse events
following IV LOCM administration to patients with pheochromocytomas and paragangliomas.
Treatment
The proper treatment of an acute contrast reaction varies depending on the presentation. A variety of scenarios and possible
treatment algorithms are discussed in Tables 2 and 3.
Delayed Adverse Events to Iodinated Contrast Media
Timing
Delayed allergic-like and non-allergic-like adverse events that occur following ICM exposure have long been a source of
concern. Such reactions are most commonly cutaneous and may develop from 30 to 60 minutes to up to one week following
contrast material exposure, with the majority occurring between three hours and two days [25,33].
ALLERGIC-LIKE AND PHYSIOLOGIC REACTIONS TO INTRAVASCULAR IODINATED CONTRAST MEDIA 29
Incidence
The incidence of delayed allergic-like reactions has been reported to range from 0.5% to 14% [33,34]. A prospective study
of 258 individuals receiving intravenous iohexol demonstrated a delayed reaction rate of 14.3% compared to 2.5% in a
control group undergoing imaging without intravascular contrast material [34]. In that same study, 26 of 37 delayed adverse
reactions were cutaneous in nature [34]. For several reasons (lack of awareness of such adverse events, usual practice
patterns, relatively low frequency of serious outcomes), such reactions are often not brought to the attention of the radiologist.
Delayed reactions are more common in patients treated with interleukin-2 (IL-2) therapy [33,35,36].
There is some evidence that the iso-osmolar dimer iodixanol may have a slightly higher rate of delayed cutaneous adverse
events when compared to other LOCM [36]. A prospective study by Schild et al [37] demonstrated an increased frequency
of delayed cutaneous adverse events to nonionic dimeric contrast material compared to nonionic monomeric contrast
material.
Symptoms
The most frequent delayed adverse events following ICM administration are allergic-like and cutaneous [2,33,34,36]. They
occur more often than is generally recognized, can recur or have serious sequelae, and are often inadvertently ascribed to
causes other than ICM.
Delayed cutaneous reactions commonly manifest as urticaria and/or a persistent rash [2,33,34,36], presenting as a
maculopapular exanthem that varies widely in size and distribution [2,25,33,38], or a generalized exanthematous pustulosis
[39]. Urticaria and/or angioedema may also occur, and is usually associated with pruritus [25,33]. Occasionally, pruritis may
occur in the absence of urticaria.
Severe cutaneous reactions have also been described in individuals with systemic lupus erythematosus (SLE) [36,40,41]. A
study by Mikkonen et al [42] suggested that delayed cutaneous adverse events may occur at an increased frequency during
certain times of the year, and most commonly affect sun-exposed areas of the body. Cases have been also reported in which
the reaction manifests similar to Stevens-Johnson syndrome [41,43], toxic epidermal necrolysis, or cutaneous vasculitis.
Rare fatalities have been described [40,41].
A variety of delayed non-cutaneous symptoms and signs have been also reported. These include nausea, vomiting, fever,
drowsiness, and headache. Severe delayed noncutaneous contrast reactions, while extremely rare, have been described,
including severe hypotension [44] and cardiopulmonary arrest; however, at least some of the events may have been due to
etiologies other than ICM.
Other Rare Delayed Adverse Events
Iodide “mumps” (iodine-related sialoadenopathy or salivary gland swelling) [45,46] and acute polyarthropathy [47] are two
additional delayed contrast reactions that have been reported rarely after ICM administration. These reactions may be more
frequent in patients with renal dysfunction.
Treatment
Since delayed reactions are generally self-limited, most require no or minimal therapy [36]. Treatment is usually supportive,
with antihistamines and/or corticosteroids used for cutaneous symptoms, antipyretics for fever, antiemetics for nausea, and
fluid resuscitation for hypotension. If manifestations are progressive or widespread, or if there are noteworthy associated
symptoms, consultation with an allergist and/or dermatologist may be helpful.
Recurrence Rates and Prophylaxis
The precise recurrence rate of delayed contrast reactions is not known but anecdotally may be 25% or more [36]. Based on
this tendency to recur, at least some of these reactions may be due to T cell-mediated hypersensitivity [2,33,34,36,38,48].
The efficacy of corticosteroid and/or antihistamine prophylaxis is unknown, though some have suggested this practice [36].
However, given the likely differing mechanisms between acute and delayed reactions, as well as the extreme rarity or
nonexistence of severe delayed reactions, premedication prior to future contrast-enhanced studies is not specifically
advocated in patients with solely a prior history of mild delayed cutaneous reaction.
ALLERGIC-LIKE AND PHYSIOLOGIC REACTIONS TO INTRAVASCULAR IODINATED CONTRAST MEDIA 30
References
1.
Bettmann MA, Heeren T, Greenfield A, Goudey C. Adverse events with radiographic contrast agents: results of the SCVIR Contrast Agent
Registry. Radiology 1997; 203:611-620.
2.
Brockow K. Contrast media hypersensitivity--scope of the problem. Toxicology 2005; 209:189-192.
3.
Bush WH, Swanson DP. Acute reactions to intravascular contrast media: types, risk factors, recognition, and specific treatment. AJR 1991;
157:1153-1161.
4.
Caro JJ, Trindade E, McGregor M. The risks of death and of severe nonfatal reactions with high - vs low-osmolality contrast media: a meta-
analysis. AJR 1991; 156:825-832.
5.
Ellis JH, Cohan RH, Sonnad SS, Cohan NS. Selective use of radiographic low-osmolality contrast media in the 1990s.
Radiology 1996; 200:297-311.
6.
Katayama H, Yamaguchi K, Kozuka T, Takashima T, Seez P, Matsuura K. Adverse reactions to ionic and nonionic contrast media. A report
from the Japanese Committee on the Safety of Contrast Media. Radiology 1990; 175:621-628.
7.
Lasser EC, Lyon SG, Berry CC. Reports on contrast media reactions: analysis of data from reports to the U.S. Food and Drug Administration.
Radiology 1997; 203:605-610.
8.
Lawrence V, Matthai W, Hartmaier S. Comparative safety of high-osmolality and low-osmolality radiographic contrast agents. Report of a
multidisciplinary working group. Invest Radiol 1992; 27:2-28.
9.
Lieberman PL, Seigle RL. Reactions to radiocontrast material. Anaphylactoid events in radiology. Clin Rev Allergy Immunol
1999; 17:469-496.
10.
Siegle RL. Rates of idiosyncratic reactions. Ionic versus nonionic contrast media. Invest Radiol 1993; 28 Suppl 5: S95-98; discussion S99.
11.
Wolf GL, Arenson RL, Cross AP. A prospective trial of ionic vs nonionic contrast agents in routine clinical practice: comparison of adverse
effects. AJR 1989; 152:939-944.
12.
Cohan RH, Dunnick NR. Intravascular contrast media: adverse reactions. AJR 1987; 149:665-670.
13.
Dunnick NR, Cohan RH. Cost, corticosteroids, and contrast media. AJR 1994; 162:527-529.
14.
Almen T. The etiology of contrast medium reactions. Invest Radiol 1994; 29 Suppl 1: S37-45.
15.
Lasser EC. A coherent biochemical basis for increased reactivity to contrast material in allergic patients: a novel concept. AJR
1987; 149:1281-1285.
16.
Bouachour G, Varache N, Szapiro N, L’Hoste P, Harry P, Alquier P. Noncardiogenic pulmonary edema resulting from intravascular
administration of contrast material. AJR 1991; 157:255-256.
17.
Cochran ST, Bomyea K, Sayre JW. Trends in adverse events after IV administration of contrast media. AJR 2001; 176:1385-1388.
18.
Mortele KJ, Oliva MR, Ondategui S, Ros PR, Silverman SG. Universal use of nonionic iodinated contrast medium for CT: evaluation of safety
in a large urban teaching hospital. AJR 2005; 184:31-34.
19.
Wang CL, Cohan RH, Ellis JH, Caoili EM, Wang G, Francis IR. Frequency, outcome, and appropriateness of treatment of nonionic iodinated
contrast media reactions. AJR 2008; 191:409-415.
20.
Dillman JR, Strouse PJ, Ellis JH, Cohan RH, Jan SC. Incidence and severity of acute allergic-like reactions to i.v. nonionic iodinated contrast
material in children. AJR 2007; 188:1643-1647.
21.
Callahan MJ, Poznauskis L, Zurakowski D, Taylor GA. Nonionic iodinated intravenous contrast material-related reactions: incidence in large
urban children’s hospital--retrospective analysis of data in 12,494 patients. Radiology 2009; 250:674-681.
22.
Curry NS, Schabel SI, Reiheld CT, Henry WD, Savoca WJ. Fatal reactions to intravenous nonionic contrast material.
Radiology 1991; 178:361-362.
23.
Spring DB, Bettmann MA, Barkan HE. Deaths related to iodinated contrast media reported spontaneously to the U.S. Food and Drug
Administration, 1978-1994: effect of the availability of low-osmolality contrast media. Radiology 1997; 204:333-337.
24.
Kopp AF, Mortele KJ, Cho YD, Palkowitsch P, Bettmann MA, Claussen CD. Prevalence of acute reactions to iopromide: postmarketing
surveillance study of 74,717 patients. Acta Radiol 2008; 49:902-911.
25.
Meth MJ, Maibach HI. Current understanding of contrast media reactions and implications for clinical management. Drug Saf
2006; 29:133-141.
26.
Davenport MS, Cohan RH, Caoili EM, Ellis JH. Repeat contrast medium reactions in premedicated patients: frequency and severit y. Radiology
2009; 253:372-379.
27.
Freed KS, Leder RA, Alexander C, DeLong DM, Kliewer MA. Breakthrough adverse reactions to low-osmolar contrast media after steroid
premedication. AJR 2001; 176:1389-1392.
28.
Lang DM, Alpern MB, Visintainer PF, Smith ST. Increased risk for anaphylactoid reaction from contrast media in patients on be ta- adrenergic
blockers or with asthma. Ann Intern Med 1991; 115:270-276.
29.
Fischer HW, Doust VL. An evaluation of pretesting in the problem of serious and fatal reactions to excretory urography.
Radiology 1972; 103:497-501.
30.
Yamaguchi K, Katayama H, Takashima T, Kozuka T, Seez P, Matsuura K. Prediction of severe adverse reactions to ionic and
nonionic contrast media in Japan: evaluation of pretesting. A report from the Japanese Committee on the Safety of Contrast Media.
Radiology 1991; 178:363-367.
31.
Somashekar DK, Davenport MS, Cohan RH, Dillman JR, Ellis JH. Effect of Intravenous Low-Osmolality Iodinated Contrast Media on Patients
with Myasthenia Gravis. Radiology 2013.
32.
Bessell-Browne R, O’Malley ME. CT of pheochromocytoma and paraganglioma: risk of adverse events with i.v. administration of nonionic
contrast material. AJR 2007; 188:970-974.
33.
Christiansen C, Pichler WJ, Skotland T. Delayed allergy-like reactions to X-ray contrast media: mechanistic considerations.
Eur Radiol 2000; 10:1965-1975.
34.
Loh S, Bagheri S, Katzberg RW, Fung MA, Li CS. Delayed adverse reaction to contrast-enhanced CT: a prospective single- center study
comparison to control group without enhancement. Radiology 2010; 255:764-771.
35.
Choyke PL, Miller DL, Lotze MT, Whiteis JM, Ebbitt B, Rosenberg SA. Delayed reactions to contrast media after interleukin -2 immunotherapy.
Radiology 1992; 183:111-114.
36.
Webb JA, Stacul F, Thomsen HS, Morcos SK. Late adverse reactions to intravascular iodinated contrast media. Eur Radiol
2003; 13:181-184.
ALLERGIC-LIKE AND PHYSIOLOGIC REACTIONS TO INTRAVASCULAR IODINATED CONTRAST MEDIA 31
37.
Schild HH, Kuhl CK, Hubner-Steiner U, Bohm I, Speck U. Adverse events after unenhanced and monomeric and dimeric contrast -enhanced CT:
a prospective randomized controlled trial. Radiology 2006; 240:56-64.
38.
Vernassiere C, Trechot P, Commun N, Schmutz JL, Barbaud A. Low negative predictive value of skin tests in investigating delayed reactions to
radio-contrast media. Contact Dermatitis 2004; 50:359-366.
39.
Peterson A, Katzberg RW, Fung MA, Wootton-Gorges SL, Dager W. Acute generalized exanthematous pustulosis as a delayed dermatotoxic
reaction to IV-administered nonionic contrast media. AJR Am J Roentgenol 2006; 187: W198-201.
40.
Goodfellow T, Holdstock GE, Brunton FJ, Bamforth J. Fatal acute vasculitis after high-dose urography with iohexol. Br J Radiol
1986; 59:620-621.
41.
Savill JS, Barrie R, Ghosh S, Muhlemann M, Dawson P, Pusey CD. Fatal Stevens-Johnson syndrome following urography with iopamidol in
systemic lupus erythematosus. Postgrad Med J 1988; 64:392-394.
42.
Mikkonen R, Vehmas T, Granlund H, Kivisaari L. Seasonal variation in the occurrence of late adverse skin reactions to iodine- based contrast
media. Acta Radiol 2000; 41:390-393.
43.
Laffitte E, Nenadov Beck M, Hofer M, Hohl D, Panizzon RG. Severe Stevens-Johnson syndrome induced by contrast medium iopentol
(Imagopaque). Br J Dermatol 2004; 150:376-378.
44.
Newman B. Delayed adverse reaction to nonionic contrast agents. Pediatr Radiol 2001; 31:597-599.
45.
Berman HL, Delaney V. Iodide mumps due to low-osmolality contrast material. AJR 1992; 159:1099-1100.
46.
Gilgen-Anner Y, Heim M, Ledermann HP, Bircher AJ. Iodide mumps after contrast media imaging: a rare adverse effect to iodine. Ann Allergy
Asthma Immunol 2007; 99:93-98.
47.
Donnelly PK, Williams B, Watkin EM. Polyarthropathy--a delayed reaction to low osmolality angiographic contrast medium in patients with
end stage renal disease. Eur J Radiol 1993; 17:130-132.
48.
Brockow K, Christiansen C, Kanny G, et al. Management of hypersensitivity reactions to iodinated contrast media. Allergy 2005; 60:150-158.
CONTRAST MEDIA WARMING 32
CONTRAST MEDIA WARMING
This chapter will discuss the relevant literature pertaining to the extrinsic warming of contrast media and provide suggestions
of cases in which extrinsic warming of contrast media may be beneficial in the care of patients.
Introduction
Contrast media viscosity, like that of many other liquids, is related to temperature. As the temperature of a given contrast
medium increases, there is a concomitant decrease in its dynamic viscosity [1]. Therefore, warmed contrast media are less
viscous than room temperature contrast media. When a warmed contrast medium is hand- or power-injected into an
intravenous (IV) or intra-arterial (IA) catheter, there will be less resistance than if the contrast medium had not been warmed.
The relationship between viscosity and flow for contrast medium injections is typically non-linear because the flow through
small bore IV catheters is turbulent and does not obey traditional laminar flow kinetics (Poiseuille’s law) [2].
Iodinated Contrast Media – Contrast Material Warming and Injection Kinetics
Several investigators have studied the effects of extrinsic warming of iodinated contrast media on IV and IA injection kinetics
[1-9].
Halsell [5] studied the in vitro flow rates through different sized angiographic catheters with and without extrinsic contrast
media warming (37
o
C). Contrast warming resulted in a flow rate improvement of 8% or more only when using high-viscosity
contrast media (a highly concentrated ionic high-osmolality monomer and an ionic low-osmolality dimer from among the
tested agents) through 4 to 5F catheters. Lower viscosity contrast media (including a nonionic monomer at 300 mg I/mL) and
larger catheters did not show this flow improvement.
Hughes and Bisset [2] measured the iodine delivery rates for a variety of low-osmolality contrast media (LOCM) at both
room (24
o
C) and human body temperature (37
o
C) and concluded that extrinsic warming to 37
o
C improved iodine delivery
rates for forceful hand injection through a 5F angiocatheter by 20% to 27% (average of 23.5%). They also found that the
iodine delivery rates closely mimicked the dynamic viscosity of the tested contrast media. Contrast media with a greater
viscosity tended to be delivered at substantially fewer milligrams of iodine per second compared to those with a lesser
viscosity. The authors suggested that vascular opacification with forceful hand injection, such as that used during catheter
angiography, could be maximized by reducing the viscosity of the utilized contrast media, either by using a lower viscosity
contrast material or by extrinsic warming.
Roth et al [3] tested four different ionic and nonionic iodinated contrast media through 12 different-sized catheters at both
human body (37
o
C) and room temperature (20
o
C), and measured the power injection pressure of each combination using a 7
mL injection at 3 mL/second with an electronic pressure transducer. Their results supported some of Halsell’s [5] findings by
showing that warmed contrast media have a lower viscosity, and this viscosity translates into a reduction in injection pressure,
but primarily for smaller diameter (< 6 French) catheters.
Busch et al [4] studied the iodine delivery rates of four different contrast media through five different catheters used for
coronary angiography at power injections of 100, 200, and 400 psi. Iodine delivery rates were treated as a surrogate for
vascular opacification. The iodine delivery rate improved with increasing pressure, increasing iodine content (mg I/mL) and
decreasing contrast media viscosity. Although the authors did not test the effect of extrinsic warming, they speculated that
the reduction in viscosity associated with warming may be a method by which iodine delivery rates might be improved. This
benefit might be greatest for lower pressure injections, such as hand injections.
Hazirolan et al [8] randomized patients undergoing cardiac CT angiography into two groups: 1) 32 patients receiving warmed
(37
o
C) iohexol 350 mg I/mL and 2) 32 patients receiving non-warmed (24
o
C) iohexol 350 mg I/mL, and then compared the
timing and degree of subsequent arterial opacification for a test bolus injection rate of 5 mL/second through an 18-gauge
peripheral IV catheter. They found that the degree of maximal enhancement within the ascending aorta, descending aorta,
and pulmonary arteries was significantly greater (p = 0.005) for group 1. They also found that group 1 patients reached 100
Hounsfield Units of enhancement within the ascending aorta significantly faster than group 2 patients (p = 0.03). The authors
concluded that extrinsic warming of the relatively viscous iohexol 350 improved the speed and degree of enhancement for
high-rate cardiac CT angiography. However, their data was solely based on the test injection (not the diagnostic injection).
CONTRAST MEDIA WARMING 33
Schwab et al [9] tested the maximum injection pressures of iopamidol 300, iomeprol 350, and iomeprol 400 at both room
(20
o
C) and human body temperature (37
o
C) through 18, 20 and 22-gauge IV catheters using a variety of injection rates (1 to
9 mL/second) with a pressure-limited (300-psi) power injector. They concluded that warming of contrast media led to
significant (p < 0.001) reductions in injection pressures across all tested media. Despite the fact that the manufacturer’s
recommended pressure thresholds were exceeded with high- rate injections (e.g., 8 mL/second), there were no instances of
IV catheter malfunction.
Iodinated Contrast Media – Contrast Material Warming and Adverse Events
Although there is good evidence that warming of contrast media changes the bolus kinetics and injection pressure of iodinated
contrast media, there has been little evidence that it affects clinical adverse event rates in a meaningful way [10-12].
In 1982, Turner et al [10] randomly assigned 100 patients in a double-blind fashion to receive either room temperature (20 to
24
o
C) or human body temperature (37
o
C) ionic high osmolality contrast media (HOCM), and then compared the
anaphylactoid and non-anaphylactoid adverse event rates between these two groups. The authors were unable to show a
significant difference, although their study was likely underpowered for a non-inferiority design. They did not report
extravasation events.
Vergara et al [11] conducted a non-randomized prospective study of 4,936 IV injections of iodinated contrast media in which
each group of patients received a specific contrast media and temperature combination. These groups were then compared
with respect to their allergic-like and physiologic adverse events. Again, extravasation rates were not assessed. The authors
showed a small but significant reduction in overall adverse events for warmed (37
o
C) ionic HOCM compared to the same
non-warmed (22
o
C) ionic HOCM (89/894 [10.0%] vs. 204/1607 [12.7%]). The dominant effect was a reduction in mild
adverse events (49/894 [5.5%] vs. 138/1607 [8.6%]) rather than a reduction in adverse events that were moderate (36/894
[4.0%] vs. 59/1607 [3.7%]) or severe (4/894 [0.45%] vs. 7/1607 [0.44%]).
Based on the above work, as well as the package inserts for many iodinated contrast media, many institutions heat their
iodinated contrast media (both HOCM and LOCM) to human body temperature (37
o
C) prior to routine clinical intravascular
administration. In most instances, this is performed using an external incubator in which the bottles of contrast media are
placed. The temperature of the device is typically kept at or near human body temperature (37
o
C). In addition to these stand-
alone warming machines, there also exist warming “sleeves” that can be used to keep pre-warmed bottles (or syringes filled
from pre-warmed bottles) of contrast media at a stable (warmed) temperature for approximately one hour or more in cases
where the contrast media is removed from the warming device but not immediately injected. These sleeves can be a
component to the power injector itself or can function independently.
Because contrast media are designated as medications, the warming of contrast media has fallen under the regulation of The
Joint Commission, which mandates that if contrast media are to be extrinsically warmed, there must be both a daily
temperature log for each warmer and evidence of regular maintenance for the warming device(s). This regulation has led
some institutions to reconsider the use of these warming devices and reevaluate whether warming iodinated contrast media
to human body temperature has a significant practical, rather than just a theoretical, benefit for IV LOCM administration.
Although some institutions have discontinued the routine use of contrast media warmers for low-rate (< 5 mL/second), non-
angiographic, non-cardiac applications, there are little published data investigating what effect this may have on patient
adverse events.
The largest study investigating the effect of extrinsic warming on IV LOCM adverse events was published in 2012 [12]. In
this non-inferiority retrospective analysis of 24,830 power-injections (< 6 mL/ second) of IV LOCM, the authors compared
the rates of allergic-like reactions and extravasations before and after the discontinuation of contrast media warming at a
single institution for both iopamidol 300 (dynamic viscosity: 8.8 centiPoise (cP) at 20°C and 4.7 cP at 37°C) and the more
viscous iopamidol 370 (dynamic viscosity: 20.9 cP at 20°C and 9.4 cP at 37°C). Discontinuation of contrast media warming
had no significant effect on the allergic-like reaction or extravasation rates of iopamidol 300. However, it did result in nearly
tripling of the extravasation rate (0.27% [five of 1851] vs. 0.87% [18 of 2074], p = 0.05) and combined allergic- like and
extravasation event rate (0.43% [eight of 1851] vs 1.25% [26 of 2074], p = 0.02) for iopamidol 370. These results suggest
that contrast media warming may not be needed for iopamidol 300 but may be needed for iopamidol 370 (and possibly other
similarly viscous contrast media) if the primary goal is to minimize contrast media-related adverse events. However, the
authors did note that there was no difference in clinical outcome between the warmed and non-warmed iopamidol 370 groups,
likely because the vast majority of extravasation events and allergic-like reactions do not result in long-term morbidity or
mortality. The authors did not have any data to permit evaluation of the effect of extrinsic contrast media warming on patient
CONTRAST MEDIA WARMING 34
comfort or physiologic (e.g., nausea, vomiting, sensation of warmth) adverse events.
Warming of Iodinated Contrast Media – Suggestions
Based on the available literature, the validity of extrinsic warmers seems predicated on the intended outcome.
Extrinsic warming of iodinated contrast material to human body temperature (37°C) may be helpful to minimize
complications and improve vascular opacification in the following circumstances:
•
For high-rate (> 5 mL/second) IV LOCM power injections
•
For injections of viscous iodinated contrast (e.g., iopamidol 370, and presumably other contrast media with a
similar or higher viscosity)
•
For direct arterial injections through small-caliber catheters (5 French or smaller)
•
For intravenously injected arterial studies in which timing and peak enhancement are critical features
Extrinsic warming of iodinated contrast material may not be needed or beneficial in the following circumstances:
•
For low-rate (≤ 5 mL/second) IV LOCM power injections or hand injections
•
For injections of iodinated contrast media with a relatively low viscosity (e.g., iopamidol 300, and presumably
other contrast media with a similar or lower viscosity)
•
For direct arterial injections through large-bore catheters (6 French or larger)
•
For IV injections in which peak opacification and timing are not critical (e.g., routine portal venous phase
chest/abdomen/pelvis CT imaging)
Package inserts for iodinated contrast media contain information about recommended storage temperatures.
Warming of Gadolinium-Based Contrast Media—Suggestions
Gadolinium-based contrast media are administered at room temperature (15 to 30
o
C [59 to 86
o
F]) and according to
package inserts, should not be externally warmed for routine clinical applications.
References
1.
Brunette J, Mongrain R, Rodes-Cabau J, et al. Comparative rheology of low- and iso-osmolarity contrast agents at different temperatures.
Cath and Cardiov Interv 2008; 71:78-83.
2.
Hughes PM, Bisset R. Non-ionic contrast media: a comparison of iodine delivery rates during manual injection angiography.
Brit J Radiol 1991; 64:417-419.
3.
Roth R, Akin M, Deligonul U, Kern MJ. Influence of radiographic contrast media viscosity to flow through coronary angiographic
catheters. Cathet Cardiovasc Diagn 1991; 22(4):290-294.
4.
Busch HP, Stocker KP. Iodine delivery rate in catheter angiography under pressure conditions in manual injection. Aktuelle Radiol
1998; 8:232-235.
5.
Halsell RD. Heating contrast media: role in contemporary angiography. Radiology 1987; 164:276-278.
6.
Pugh ND. Haemodynamic and rheological effects of contrast media: the role of viscosity and osmolality. Eur Radiol 1996; 6: S13- S15.
7.
Bae KT. Intravenous contrast medium administration and scan timing at CT: considerations and approaches. Radiology 2010; 256:32-61.
8.
Hazirolan T, Turkbey B, Akpinar E, et al. The impact of warmed intravenous contrast media on the bolus geometry of coronary C T
angiography applications. Korean J Radiol 2009; 10:150-155.
9.
Schwab SA, Kuefner MA, Anders K, et al. Peripheral intravenous power injection of iodinated contrast media: the impact of tem perature
on maximum injection pressures at different cannula sizes. Acad Radiol 2009; 16:1502-1508.
10.
Turner E, Kentor P, Melamed JL, et al. Frequency of anaphylactoid reactions during intravenous urography with radiographic contrast
media at two different temperatures. Radiology 1982; 143:327-329.
11.
Vergara M, Seguel S. Adverse reactions to contrast media in CT: effects of temperature and ionic property. Radiology 1996; 199:363-
366.
12.
Davenport MS, Wang CL, Bashir MR, et al. Rate of contrast media extravasations and allergic -like reactions: effect of extrinsic warming
of low-osmolality iodinated CT contrast media to 37oC. Radiology 2012; 262:475-484.
POST-CONTRAST ACUTE KIDNEY INJURY AND CONTRAST-INDUCED NEPHROPATHY IN ADULTS 35
POST-CONTRAST ACUTE KIDNEY INJURY AND CONTRAST-INDUCED
NEPHROPATHY IN ADULTS
Definitions and Terminology
Contrast-associated acute kidney injury (CA-AKI) (formerly known as post-contrast acute kidney injury (PC-AKI)) is a general
term used to describe a sudden deterioration in renal function that occurs within 48 hours following the intravascular
administration of iodinated contrast medium. CA-AKI may occur regardless of whether the contrast medium was the cause of the
deterioration [1-12]. CA-AKI is a correlative diagnosis.
Contrast-induced acute kidney injury (CI-AKI) (formerly known as contrast-induced nephropathy (CIN) is a specific term used
to describe a sudden deterioration in renal function that is caused by the intravascular administration of iodinated contrast medium;
therefore, CI-AKI is a subgroup of CA-AKI [1-12]. CI-AKI is a causative diagnosis.
Unfortunately, very few published studies have a suitable control group to permit the separation of CI-AKI from CA-AKI [1-12].
Therefore, the incidence of CA-AKI reported in clinical studies and the incidence of CA-AKI observed in clinical practice likely
includes a combination of CI-AKI (i.e., AKI caused by contrast medium administration) and AKI unrelated to contrast medium
administration (i.e., AKI coincident to but not caused by contrast medium administration).
This document will address both CI-AKI and CA-AKI, but these terms are not interchangeable. CA-AKI is not synonymous with
CI-AKI.
At the current time, it is the position of ACR Committee on Drugs and Contrast Media that CI-AKI is a real, albeit rare,
entity. Published studies on CI-AKI have been heavily contaminated by bias and conflation. Future investigations building on
recent methodological advancements [3,4,7,9], are necessary to clarify the incidence and significance of this disease.
Pathogenesis
CA-AKI may be caused by any nephrotoxic event (including CI-AKI) that is coincident to the intravascular administration of
contrast material. Because the diagnosis of CA-AKI is based on changes in serum creatinine [2,13-15] physiologic fluctuation in
this value can also contribute to its incidence, particularly in patients with chronic kidney disease. Patients who have an elevated
serum creatinine at baseline have a greater variance in daily serum creatinine measurements than those with a normal baseline
serum creatinine [10].
The exact pathophysiology of CI-AKI is not understood. Etiologic factors that have been suggested include renal hemodynamic
changes (vasoconstriction) and direct tubular toxicity, among others [16-26]. Both osmotic and chemotoxic mechanisms may be
involved, and some investigations suggest agent-specific chemotoxicity. The nephrotoxic effect of iodinated contrast medium
may be proportional to dose for cardiac angiography; there is no evidence of a dose-toxicity relationship following intravenous
(IV) administration when administered at usual diagnostic doses. CI-AKI may occur in children, but if so, it is rare [27-30].
Gadolinium-based contrast media either do not cause CI-AKI when administered at FDA-approved doses, or this event is
exceptionally rare [31-35]. If administered at extreme above-FDA-label doses to achieve X-ray attenuating effects during
angiography (not recommended), gadolinium-based contrast media are more nephrotoxic than iso-attenuating doses of iodinated
contrast media [36-38].
Diagnosis
Kidney Disease Improving Global Outcomes (KDIGO) criteria are recommended for the diagnosis of CA-AKI and endorsed by
the NKF Kidney Disease Outcomes Quality Initiative as a consensus definition for epidemiologic and clinical research
applications [39-41]. The 2012 Kidney Disease: Improving Global Outcomes (KDIGO) Clinical Practice Guidelines for acute
kidney injury were developed to assist practitioners caring for patients at risk for or with AKI following an explicit process of
evidence review and appraisal [40,42].
KDIGO Definition of Acute Kidney Injury
The diagnosis of AKI is made according to the KDIGO criteria if one of the following occurs within 48 hours after a nephrotoxic
event (e.g., intravascular iodinated contrast medium exposure) [40]:
1.
Absolute serum creatinine increase ≥0.3 mg/dL (>26.4 µmol/L).
2.
A percentage increase in serum creatinine ≥ 50% (≥1.5-fold above baseline).
POST-CONTRAST ACUTE KIDNEY INJURY AND CONTRAST-INDUCED NEPHROPATHY IN ADULTS 36
3.
Urine output reduced to ≤ 0.5 mL/kg/hour for at least 6 hours.
This system has been advocated as a common definition of intrinsic acute kidney injury, regardless of etiology [40]. Therefore, it
can be used to define the parameters of CA-AKI as well as CI-AKI. The KDIGO criteria also outline a system for staging the
degree of renal injury that is present following the diagnosis of AKI; the interested reader is referred to the original manuscript
[40].
Elevations in serum creatinine are neither sensitive nor specific for individual types of AKI. Any serum creatinine-based criteria,
used in isolation, will be unable to separate CI-AKI from generic CA-AKI. This applies to scientific studies lacking appropriate
control groups and to clinical evaluations of individual patients [2-4,7-9,11].
Laboratory Tests of Renal Function
Laboratory tests may be used both to estimate the risk of CI-AKI prior to administering contrast medium and to determine whether
AKI has occurred after contrast medium administration. Serum creatinine concentration is the most commonly used measure of
renal function, but it has limitations as an accurate measure of glomerular filtration rate (GFR) [43 -47]. Serum creatinine is
considerably influenced by the patient’s gender, muscle mass, nutritional status, and age. Impaired renal function can exist when
the serum creatinine is “normal”. Normal serum creatinine is maintained until the GFR – at least as reflected in creatinine clearance
– is reduced by nearly 50%.
Calculated estimated glomerular filtration rate (eGFR) is more accurate than is serum creatinine at predicting true GFR [48]. As
a result, eGFR is gaining attention as a potentially better marker of CI-AKI risk [49,50].
Although the formula for estimating GFR has intrinsic error due to the reliance on serum creatinine, and lack of validation in
patients with AKI, low muscle mass or patients on dialysis, it is the most accurate and least biased method commonly available
to stratify KDIGO CKD stages.
Route of Contrast Administration
In the last two decades, the CI-AKI literature has been dominated by reports of patients who have undergone cardiac angiography
with iodinated contrast medium. Cardiac angiography differs from IV contrast medium administration in three major ways: 1) the
injection is intra-arterial and supra-renal, 2) the injection requires a catheter that can dislodge atheroemboli, and 3) the contrast
medium dose to the kidneys will be more abrupt and concentrated [2,6,51,52].
The overall incidence of CA-AKI in studies of cardiac angiography is higher than it is in studies of patients who receive IV
iodinated contrast medium. Therefore, data from cardiac angiography studies likely over-estimate the risk of CI-AKI for patients
undergoing IV contrast-enhanced studies [2,6].
CI-AKI Studies
Much of the literature investigating the incidence of CI-AKI has failed to include a control group of patients not receiving contrast
medium [8,12]. This is problematic because several studies have shown that the frequency and magnitude of serum creatinine
change in patients who have not received contrast medium is similar to the changes in patients who have received it [7 -9,53-60].
In more than 30,000 patients at a single institution who did not receive any contrast medium, more than half showed a change in
serum creatinine of at least 25%, and more than 40% showed a change of at least 0.4 mg/dL [10]. The authors noted that had some
of these patients received iodinated contrast medium temporally related to the rise in serum creatinine, the rise would have been
undoubtedly attributed to it, rather than to physiologic variation or another etiology.
Since 2007, an increasing number of published studies have included control groups of patients not exposed to iodinated contrast
medium [53,55-60]. Most have found no evidence of CI-AKI, but most also utilized non- randomized non-matched controls who
happened to receive unenhanced CT as part of routine clinical care [53,55-60]. The clinical population of patients imaged with
unenhanced CT is enriched with patients who are at risk for AKI and therefore is contaminated by selection bias. This selection
bias has been shown objectively in a meta-analysis by McDonald et al [8].
Four large studies released in 2013 and 2014 (each with >10,000 patients) have addressed selection bias in the unenhanced CT
population through use of propensity score adjustment and propensity score matching [3,4,7,9]. Although the conclusions from
these studies differ somewhat, all four have shown that CI-AKI is much less common than previously believed. In patients with
a stable baseline eGFR ≥45 mL / min/1.73m2, IV iodinated contrast media are not an independent nephrotoxic risk factor [3,4,7,9],
and in patients with a stable baseline eGFR 30-44 mL / min/1.73m2, IV iodinated contrast media are either not nephrotoxic or
POST-CONTRAST ACUTE KIDNEY INJURY AND CONTRAST-INDUCED NEPHROPATHY IN ADULTS 37
rarely so [3,4,7,9].
Despite this common ground, there are differences among these studies [3,4,7,9] in the covariates chosen for inclusion, the method
of controlling baseline renal function instability, the definitions of AKI, and the nuances of the statistical methodology. These
differences likely explain the different conclusions drawn between these studies for patients with Stage IV and Stage V chronic
kidney disease (eGFR <30 mL / min/1.73m2). In particular, two propensity-score matched studies [3,4] have shown that IV
iodinated contrast material is an independent nephrotoxic risk factor in patients with Stage IV and Stage V chronic kidney disease,
while two others were unable to find such evidence [7,9].
Risk Factors
Numerous studies have attempted to isolate risk factors for CI-AKI. There is consensus that the most important risk factor is pre-
existing severe renal insufficiency [3,4,15,61,62]. Multiple other risk factors have been proposed, including diabetes mellitus,
dehydration, cardiovascular disease, diuretic use, advanced age, hypertension, hyperuricemia, and multiple iodinated contrast
medium doses in a short time interval (<24 hours) [3,4,15,61-64], but these have not been rigorously confirmed. In particular,
multiple myeloma is not supported as a risk factor for CA-AKI [65-67]. Two studies have shown that CA-AKI may occur after
two closely spaced doses of IV iodinated contrast medium [63,64], but neither study was designed to show that the risk was higher
than after one or no dose of IV contrast medium.
Risk Thresholds
There is no agreed-upon threshold of serum creatinine elevation or eGFR declination beyond which the risk of CI-AKI is
considered so great that intravascular iodinated contrast medium should never be administered. In fact, since each contrast medium
administration always implies a risk-benefit analysis for the patient, contrast medium administration for all patients should always
be taken in the clinical context, considering all risks, benefits and alternatives [2,6].
Some practices have advocated stratification of potential risk by eGFR instead of serum creatinine because it is a better indicator
of baseline renal function [49,50]. This has been limited in the past by insufficient data [68-70], but there are now two large
propensity score-adjusted studies that stratify CI-AKI risk by eGFR [3,7]. One showed no risk of CI-AKI from IV iodinated
contrast material, regardless of baseline eGFR [7], while another identified patients with an eGFR <30 mL / min/1.73m2 to be at
significant risk (patients with eGFR 30-44 mL / min/1.73m2 were at borderline but not statistically significant risk) [3].
Herts et al [50] showed that when patients’ eGFR was calculated by the MDRD formula, a significantly higher percentage of
patients presenting for contrast-enhanced CT scans had an eGFR <60 mL / min than had a serum creatinine of >1.4 mg/dL.
Davenport et al [49] showed that the use of eGFR thresholds (instead of serum creatinine-based thresholds) more appropriately
identified patients who may be at risk for CI-AKI.
At the current time, there is very little evidence that IV iodinated contrast material is an independent risk factor for AKI in patients
with eGFR ≥30 mL / min/1.73m2. Therefore, if a threshold for CI-AKI risk is used at all, 30 mL / min/1.73m2 seems to be the
one with the greatest level of evidence [3]. Any threshold put into practice must be weighed on an individual patient level w ith
the benefits of administering contrast material.
Contrast-enhanced CT has superior diagnostic performance compared to unenhanced CT for a wide array of indications. Failure
to diagnose an important clinical entity carries its own risk.
As previously stated, no serum creatinine or eGFR threshold is adequate to stratify risk for patients with AKI because serum
creatinine in this setting is unreliable. However, in patients with AKI, the administration of iodinated contrast medium should
only be undertaken with appropriate caution, and only if the benefit to the patient outweighs the risk. There have been no published
series demonstrating that IV iodinated contrast medium administration to patients with AKI leads to worse or prolonged renal
dysfunction than would occur in a control group. However, patients with AKI are particularly susceptible to nephrotoxin exposure
and therefore it is probably prudent to avoid intravascular iodinated contrast medium in these patients when possible.
Anuric patients with end-stage renal disease who do not have a functioning transplant kidney are not at risk for CI-AKI because
their kidneys are nonfunctional; these patients may receive intravascular iodinated contrast material without risk of additional
renal injury (see Renal Dialysis Patients and the Use of Iodinated Contrast Medium, below).
Screening
Screening based on eGFR should be used to identify patients at potential risk of CI-AKI [61,71] . A variety of patient risk factors

POST-CONTRAST ACUTE KIDNEY INJURY AND CONTRAST-INDUCED NEPHROPATHY IN ADULTS 38
have been investigated as screening data elements which vary in their sensitivity and specificity, although the most useful may be
a personal history of kidney disease (i.e. CKD, remote AKI, kidney surgery, kidney ablation, albumineria) [72,73]. Diabetes
mellitus may be an additional optional factor for screening [72,73]. Patient age and treated or untreated hypertension as
independent triggers for kidney function assessment may result in a large false-positive rate in identifying patients with eGFR less
than 30 mL/min/1.73 m2.
There is no agreed-upon acceptable maximum interval between baseline renal function assessment and contrast medium
administration in at-risk patients. Some accept a 30-day interval in outpatients. It seems prudent to have a shorter interval for
inpatients, those with a new risk factor, and those with a heightened risk of renal dysfunction.
Suggested Indications for Renal Function Assessment before the Intravascular Administration of Iodinated Contrast Medium
The following is a suggested list of risk factors that may warrant renal function assessment (e.g., serum creatinine, eGFR) prior
to the administration of intravascular iodinated contrast medium. This list should not be considered definitive and represents a
blend of published data [73,74] and expert opinion:
•
Personal history of renal disease, including:
Known chronic kidney disease (CKD)
Remote history of AKI
Dialysis
Kidney surgery
Kidney ablation
Albuminuria
•
History of diabetes mellitus (optional)
•
Metformin or metformin-containing drug combinations
1
Patients who are scheduled for a routine intravascular study but do not have one of the above risk factors do not require a baseline
serum creatinine determination before iodinated contrast medium administration.
Morbidity and Mortality
The clinical course of CA-AKI (and, presumably, CI-AKI) depends on baseline renal function, coexisting risk factors, degree
of hydration, and other factors. However, the usual course consists of a transient asymptomatic elevation in serum creatinine.
Serum creatinine usually begins to rise within 24 hours of intravascular iodinated contrast medium administration, peaks
within 4 days, and often returns to baseline within 7 to 10 days. It is unusual for patients to develop permanent renal
dysfunction [68,70].
Several studies have shown that patients with CA-AKI, including those with only transient injury, tend to have longer hospital
stays, higher mortality, and higher incidences of cardiac and neurologic events than contrast medium-receiving patients whose
kidney function remains stable [41,75-78]. These observations have led to widespread hesitance in the use of intravascular
iodinated contrast medium when the risk of CI-AKI is felt to be high. However, many studies investigating CI-AKI and its
consequences following intravascular iodinated contrast medium administration have failed to include a control group of patients
not receiving contrast medium [76-78]; therefore, it is possible that much of the morbidity and mortality previously attributed to
CI-AKI in the literature may in fact be due to other etiologies (i.e., contrast-independent causes of CA-AKI). Larger studies with
proper control groups and longitudinal outcomes data are needed.
Prevention
Prior to contrast medium administration, adequate patient assessment and communication between radiologist and referring
clinician are important. Consideration of alternative imaging strategies and an individualized risk-benefit assessment are
fundamental.
Avoidance of Iodinated Contrast Medium
Concern for the development of CI-AKI is a relative but not absolute contraindication to the administration of intravascular
iodinated contrast medium in at-risk patients that have AKI or an eGFR less than 30 mL/min/1.73 m2 and are not undergoing
1
Metformin does not confer an increased risk of CI-AKI. However, patients who develop AKI while taking metformin may be susceptible to
the development of lactic acidosis.
POST-CONTRAST ACUTE KIDNEY INJURY AND CONTRAST-INDUCED NEPHROPATHY IN ADULTS 39
maintenance dialysis [3,4,70,79,80]. In these scenarios, the information that may be obtained by using no contrast medium (e.g.,
non-contrast CT) and/or other modalities (e.g., ultrasound, non-contrast magnetic resonance imaging [MRI]) may be sufficiently
useful that contrast medium administration can be avoided. (See the Chapter on Nephrogenic Systemic Fibrosis [NSF] for a full
discussion of the use of gadolinium chelates in patients with renal disease.) In some clinical situations, the use of intravascular
iodinated contrast medium may be necessary regardless of CI-AKI risk. Although there is data suggesting a directly proportional
dose- toxicity relationship for intracardiac iodinated contrast medium [81], there is no analogous robust data for intravenous
iodinated contrast media within the range of clinically administered doses. Therefore, it is not recommended to reduce doses to
attempt to mitigate the risk of CI-AKI as this may result in suboptimal or nondiagnostic images. Instead, standard contrast dosing
is recommended if the benefits have been deemed to outweigh the risks for intravenous iodinated contrast media administration
in high-risk patients for CI-AKI.
One purported risk factor for the development of CI-AKI is the administration of multiple doses of intravascular iodinated contrast
medium within a short period of time [63,64]. Most low-osmolality iodinated contrast media have a half-life of approximately
two hours. Therefore, it takes approximately 20 hours for one administered dose of contrast medium to be eliminated in a patient
with normal renal function. Therefore, it has long been suggested that dosing intervals shorter than 24 hours be avoided except in
urgent situations.
We do not believe that there is sufficient evidence to specifically endorse the decision to withhold a repeat contrast medium
injection until more than 24 hours have passed since the prior injection, nor to recommend a specific threshold of contrast medium
volume beyond which additional contrast media should not be given within a 24-hour period.
Further, obtaining a serum creatinine measurement between two closely spaced iodinated contrast medium enhanced studies are
unlikely to be of any benefit given the slow nature of change of serum creatinine in patients with AKI.
Therefore, the decision to administer closely spaced contrast-enhanced studies is clinical and subjective, with high-risk patients
(e.g., Stage IV and Stage V chronic kidney disease, AKI) treated with greater caution than the general population.
Choice of Iodinated Contrast Medium
Barrett and Carlisle [82] reported a meta-analysis of the literature concerning the relative nephrotoxicity of high osmolality
contrast media (HOCM) and low osmolality contrast media (LOCM). They concluded that LOCM are less nephrotoxic than
HOCM in patients with underlying renal insufficiency. LOCM were not shown to be significantly different in patients with normal
renal function. Most centers no longer use intravascular HOCM due to the greater incidence of various adverse effects associated
with its use.
Studies [83-86] have failed to establish a clear advantage of IV iso-osmolality iodixanol over IV LOCM with regard to CA-AKI
or CI-AKI. A 2009 meta-analysis using data pooled from 25 trials found no difference in the rate of CA-AKI between iodixanol
and low osmolality agents after intravenous administration [87].
Volume Expansion
The major preventive action to mitigate the risk of CI-AKI is to provide intravenous volume expansion prior to contrast medium
administration [88-94]. The ideal infusion rate and volume is unknown, but isotonic fluid such as 0.9% normal saline (NS) is
preferred. Typical prophylaxis regiments begin 1 hour prior to the exam and continue 3-12 hours after with longer regiments
(approximately 12 hours) shown to lower the risk of CA-AKI compared with shorter regiments [61,95]. Typical doses may be
fixed volume (e.g., 500 mL NS) before and after or weight-based volumes (1-3mL/kg per hour) [61,79]. Oral hydration has not
been well studied for patients with eGFR less than 30 mL/min/1.73 m2 or in patients with AKI.
Not all clinical studies have shown dehydration to be a major risk factor for CA-AKI. However, in the dehydrated state, renal
blood flow and GFR are decreased, the effect of iodinated contrast medium on these parameters is accentuated, and there is a
theoretical concern of prolonged tubular exposure to iodinated contrast medium due to low tubular flow rates. Solomon et al [96]
[86] studied adult patients with chronic kidney disease who underwent cardiac angiography. The reported incidence of CA -AKI
was decreased by periprocedural IV volume expansion (0.45% or 0.9% saline, 100 mL/h, 12 hours before to 12 hours after
intravascular contrast medium administration). In another study, IV volume expansion with 0.9% saline was superior to IV volume
expansion with 0.45% saline in CA-AKI risk reduction [89].
Prophylaxis is indicated for patients who have AKI or severe CKD with an eGFR less than 30 mL/min/1.73m2, although the risks
of volume expansion (i.e., heart failure or other hypervolemic conditions) should be considered before initiation [97,98].

POST-CONTRAST ACUTE KIDNEY INJURY AND CONTRAST-INDUCED NEPHROPATHY IN ADULTS 40
Prophylaxis is not indicated for the general population of patients with stable eGFR greater than or equal to 30 mL/min 1.73 m2
or patients on chronic dialysis [98]. Prophylaxis may also be considered on an individual basis for high-risk circumstances (e.g.
numerous risk factors, recent AKI, borderline eGFR) in patients with an eGFR of 30-44 mL/min./1.73 m2 at the discretion of the
ordering provider.
Sodium bicarbonate
Some studies and meta-analyses of patients undergoing cardiac angiography have shown intravenous volume expansion with
sodium bicarbonate to be superior to 0.9% saline in reducing the risk of CA-AKI [90,91], but these results have been challenged
by other meta-analyses and studies [93,99]. Bicarbonate is likely similar to normal saline for the prevention of CA-AKI, but it is
not preferred due to the additional requirement for pharmacist compounding.
N-acetylcysteine
Recent randomized trial showed that N-acetylcysteine was no more effective than placebo at preventing CA-AKI for intra-arterial
iodinated contrast media administration and is therefore not recommended for intravenous contrast media prophylaxis [99].
Diuretics: Mannitol and Furosemide or Other Agents
Solomon et al [100] reported no beneficial effects from the osmotic diuretic mannitol when it was added to IV saline solution in
patients with or without diabetes mellitus. There was an exacerbation of renal dysfunction when the loop diuretic furosemide was
used in addition to IV saline solution. Neither mannitol nor furosemide is recommended for CI-AKI risk reduction. Other
theoretical renal-protective medications such as theophylline, endothelin-1, and fenoldopam are also not recommended for CI-
AKI reduction as strong supporting data is lacking.
Renal Dialysis Patients and the Use of Iodinated Contrast Medium
Patients with anuric end-stage chronic kidney disease who do not have a functioning transplant can receive intravascular iodinated
contrast medium without risk of further renal damage because their kidneys are no longer functioning. However, there is a
theoretical risk of converting an oliguric patient on dialysis to an anuric patient on dialysis by exposing him or her to intravascular
iodinated contrast medium. This remains speculative, as there are no conclusive outcomes data in this setting. Therefore, patients
undergoing dialysis who make more than 1-2 cups of urine/day (100 mL) should be considered nonanuric and treated as high-risk
patients similar to patients with AKI or eGFR less than 30 mL/min/1.73m2 who are not undergoing hemodialysis.
Patients should not have acute dialysis nor continuous renal replacement therapy initiated or alter their schedule solely based on
iodinated contrast media administration regardless of renal function due to the risks, costs and lack of benefit [39,61,72,79,80,101-
103].
The U.S. Food and Drug Administration (FDA) has issued guidelines and drug labeling for metformin since 1995, and the component of these FDA
guidelines related to administration of iodinated contrast material in patients taking metformin has been made progressively less rigorous since the original
version. The ACR Committee on Drugs and Contrast Media recognizes that the latest (as of this writing, dated 4-8-2016) FDA guidelines and drug labeling
are still more restrictive than those in this chapter of the ACR Manual on Contrast Media. Nevertheless, the committee authoring this Manual has reviewed
the evidence and believes that the prevailing weight of clinical evidence on this matter allows less stringent yet safe patient management which should
reduce patient cost and inconvenience. This footnote is designed to alert readers that the ACR recommendations differ in case their personal philosophy or
institutional policies necessitate adherence to the more restrictive FDA guidelines.
References
1. Baumgarten DA, Ellis JH. Contrast-induced nephropathy: contrast material not required? AJR Am J Roentgenol 2008;191:383-6.
2. Davenport MS, Cohan RH, Khalatbari S, Ellis JH. The challenges in assessing contrast-induced nephropathy: where are we now? JR Am J
Roentgenol 2014;202:784-9.
3. Davenport MS, Khalatbari S, Cohan RH, Dillman JR, Myles JD, Ellis JH. Contrast material-induced nephrotoxicity and intravenous low-
osmolality iodinated contrast material: risk stratification by using estimated glomerular filtration rate. Radiology 2013;268:719-28.
4. Davenport MS, Khalatbari S, Dillman JR, Cohan RH, Caoili EM, Ellis JH. Contrast material-induced nephrotoxicity and intravenous low-
osmolality iodinated contrast material. Radiology 2013;267:94-105.
5. Ellis JH, Cohan RH. Reducing the risk of contrast-induced nephropathy: a perspective on the controversies. AJR Am J Roentgenol
2009;192:1544-9.
6. Katzberg RW, Newhouse JH. Intravenous contrast medium-induced nephrotoxicity: is the medical risk really as great as we have come to
believe? Radiology 2010;256:21-8.
7. McDonald JS, McDonald RJ, Carter RE, Katzberg RW, Kallmes DF, Williamson EE. Risk of intravenous contrast material-mediated acute
kidney injury: a propensity score-matched study stratified by baseline-estimated glomerular filtration rate. Radiology 2014;271:65-73.
8. McDonald JS, McDonald RJ, Comin J, et al. Frequency of acute kidney injury following intravenous contrast medium administration: a
systematic review and meta-analysis. Radiology 2013;267:119-28.
9. McDonald RJ, McDonald JS, Bida JP, et al. Intravenous contrast material-induced nephropathy: causal or coincident phenomenon? Radiology
2013;267:106-18.
POST-CONTRAST ACUTE KIDNEY INJURY AND CONTRAST-INDUCED NEPHROPATHY IN ADULTS 41
10. Newhouse JH, Kho D, Rao QA, Starren J. Frequency of serum creatinine changes in the absence of iodinated contrast material: implications for
studies of contrast nephrotoxicity. AJR Am J Roentgenol 2008;191:376-82.
11. Newhouse JH, RoyChoudhury A. Quantitating contrast medium-induced nephropathy: controlling the controls. Radiology 2013;267:4-8.
12. Rao QA, Newhouse JH. Risk of nephropathy after intravenous administration of contrast material: a critical literature analysi s. Radiology
2006;239:392-7.
13. Aurelio A, Durante A. Contrast-induced nephropathy in percutaneous coronary interventions: pathogenesis, risk factors, outcome, prevention and
treatment. Cardiology 2014;128:62-72.
14. Slocum NK, Grossman PM, Moscucci M, et al. The changing definition of contrast-induced nephropathy and its clinical implications: insights
from the Blue Cross Blue Shield of Michigan Cardiovascular Consortium (BMC2). American heart journal 2012;163:829-34.
15. Stacul F, van der Molen AJ, Reimer P, et al. Contrast induced nephropathy: updated ESUR Contrast Media Safety Committee guide lines.
European radiology 2011;21:2527-41.
16. Deray G, Dubois M, Martinez F, et al. Renal effects of radiocontrast agents in rats: a new model of acute renal failure. Amer ican journal of
nephrology 1990;10:507-13.
17. Gruskin AB, Oetliker OH, Wolfish NM, Gootman NL, Bernstein J, Edelmann CM, Jr. Effects of angiography on renal function and h istology in
infants and piglets. The Journal of pediatrics 1970;76:41-8.
18. Heinrich MC, Kuhlmann MK, Grgic A, Heckmann M, Kramann B, Uder M. Cytotoxic effects of ionic high -osmolar, nonionic monomeric, and
nonionic iso-osmolar dimeric iodinated contrast media on renal tubular cells in vitro. Radiology 2005;235:843-9.
19. Heneghan M. Contrast-induced acute renal failure. AJR Am J Roentgenol 1978;131:1113-5.
20. Katzberg RW, Morris TW, Schulman G, et al. Reactions to intravenous contrast media. Part II: Acute renal response in euvolemic and dehydrated
dogs. Radiology 1983;147:331-4.
21. Liu ZZ, Viegas VU, Perlewitz A, et al. Iodinated contrast media differentially affect afferent and efferent arteriolar tone and reactivity in mice: a
possible explanation for reduced glomerular filtration rate. Radiology 2012;265:762-71.
22. Lund G, Einzig S, Rysavy J, et al. Role of ischemia in contrast-induced renal damage: an experimental study. Circulation 1984;69:783-89.
23. Porter GA, Kloster FE, Bristow JD. Sequential effect of angiographic contrast agent on canine renal and systemic hemodynamics. American heart
journal 1971;81:80-92.
24. Sendeski M, Patzak A, Pallone TL, Cao C, Persson AE, Persson PB. Iodixanol, constriction of medullary descending vasa recta, and risk for
contrast medium-induced nephropathy. Radiology 2009;251:697-704.
25. Tadavarthy SM, Castaneda W, Amplatz K. Redistribution of renal blood flow caused by contrast media. Radiology 1977;122:343-8.
26. Ziegler TW, Ludens JH, Fanestil DD, Talner LB. Inhibition of active sodium transport by radiographic contrast media. Kidney international
1975;7:68-76.
27. Ajami G, Derakhshan A, Amoozgar H, et al. Risk of nephropathy after consumption of nonionic contrast media by children undergoing cardiac
angiography: a prospective study. Pediatric cardiology 2010;31:668-73.
28. Haight AE, Kaste SC, Goloubeva OG, Xiong XP, Bowman LC. Nephrotoxicity of iopamidol in pediatric, adolescent, and young adult patients
who have undergone allogeneic bone marrow transplantation. Radiology 2003;226:399-404.
29. Noyan A, Küçükosmanoğlu O, Yildizdaş D, Ozbarlas N, Anarat A, Anarat R. Evaluation of renal functions in children with congenital heart
disease before and after cardiac angiography. The Turkish journal of pediatrics 1998;40:97-101.
30. Senthilnathan S, Gauvreau K, Marshall AC, Lock JE, Bergersen L. Contrast administration in pediatric cardiac catheterization: dose and adverse
events. Catheterization and cardiovascular interventions : official journal of the Society for Cardiac Angiog raphy & Interventions 2009;73:814-
20.
31. Arsenault TM, King BF, Marsh JW, Jr., et al. Systemic gadolinium toxicity in patients with renal insufficiency and renal failure: retrospective
analysis of an initial experience. Mayo Clinic proceedings 1996;71:1150-4.
32. Hoffmann U, Fischereder M, Reil A, Fischer M, Link J, Krämer BK. Renal effects of gadopentetate dimeglumine in patients with normal and
impaired renal function. European journal of medical research 2005;10:149-54.
33. Prince MR, Arnoldus C, Frisoli JK. Nephrotoxicity of high-dose gadolinium compared with iodinated contrast. Journal of magnetic resonance
imaging : JMRI 1996;6:162-6.
34. Tombach B, Bremer C, Reimer P, et al. Renal tolerance of a neutral gadolinium chelate (gadobutrol) in patients with chronic renal failure: results
of a randomized study. Radiology 2001;218:651-7.
35. Zhang HL, Ersoy H, Prince MR. Effects of gadopentetate dimeglumine and gadodiamide on serum calcium, magnesium, and creatinin e
measurements. Journal of magnetic resonance imaging : JMRI 2006;23:383-7.
36. Briguori C, Colombo A, Airoldi F, et al. Gadolinium-based contrast agents and nephrotoxicity in patients undergoing coronary artery procedures.
Catheterization and cardiovascular interventions : official journal of the Society for Cardiac Angiography & Interventions 2006;67:175-80.
37. Elmståhl B, Nyman U, Leander P, Chai CM, Frennby B, Almén T. Gadolinium contrast media are more nephrotoxic than a low osmola r iodine
medium employing doses with equal X-ray attenuation in renal arteriography: an experimental study in pigs. Academic radiology 2004;11:1219-
28.
38. Erley CM, Bader BD, Berger ED, et al. Gadolinium-based contrast media compared with iodinated media for digital subtraction angiography in
azotaemic patients. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European
Renal Association 2004;19:2526-31.
39. Palevsky PM, Liu KD, Brophy PD, et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for acute kidney injury.
American journal of kidney diseases : the official journal of the National Kidney Foundation 2013;61:649-72.
40. Section 2: AKI Definition. Kidney Int Suppl (2011) 2012;2:19-36.
41. Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Critical
care (London, England) 2007;11:R31.
42. Kellum JA, Lameire N. Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1). Critical care ( London,
England) 2013;17:204.
43. Endre ZH, Pickering JW. Outcome definitions in non-dialysis intervention and prevention trials in acute kidney injury (AKI). Nephrology
Dialysis Transplantation 2009;25:107-18.
44. Charlton JR, Portilla D, Okusa MD. A basic science view of acute kidney injury biomarkers. Nephrology, dialysis, transplantat ion : official
publication of the European Dialysis and Transplant Association - European Renal Association 2014;29:1301-11.

POST-CONTRAST ACUTE KIDNEY INJURY AND CONTRAST-INDUCED NEPHROPATHY IN ADULTS 42
45. de Geus HR, Betjes MG, Bakker J. Biomarkers for the prediction of acute kidney injury: a narrative review on current status and future
challenges. Clinical kidney journal 2012;5:102-08.
46. Waikar SS, Betensky RA, Emerson SC, Bonventre JV. Imperfect gold standards for kidney injury biomarker evaluation. Journal of the American
Society of Nephrology : JASN 2012;23:13-21.
47. Ostermann M, Bellomo R, Burdmann EA, et al. Controversies in acute kidney injury: conclusions from a Kidney Disease: Improvin g Global
Outcomes (KDIGO) Conference. Kidney international 2020;98:294-309.
48. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum
creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Annals of internal medicine 1999;130:461-70.
49. Davenport MS, Khalatbari S, Cohan RH, Ellis JH. Contrast medium-induced nephrotoxicity risk assessment in adult inpatients: a comparison of
serum creatinine level- and estimated glomerular filtration rate-based screening methods. Radiology 2013;269:92-100.
50. Herts BR, Schneider E, Poggio ED, Obuchowski NA, Baker ME. Identifying outpatients with renal insufficiency before contrast -enhanced CT by
using estimated glomerular filtration rates versus serum creatinine levels. Radiology 2008;248:106-13.
51. Karlsberg RP, Dohad SY, Sheng R. Contrast medium-induced acute kidney injury: comparison of intravenous and intraarterial administration of
iodinated contrast medium. Journal of vascular and interventional radiology : JVIR 2011;22:1159-65.
52. Nyman U, Almén T, Jacobsson B, Aspelin P. Are intravenous injections of contrast media really less nephrotoxic than intra-arterial injections?
European radiology 2012;22:1366-71.
53. Bruce RJ, Djamali A, Shinki K, Michel SJ, Fine JP, Pozniak MA. Background fluctuation of kidney function versus contrast -induced
nephrotoxicity. AJR Am J Roentgenol 2009;192:711-8.
54. Cramer BC, Parfrey PS, Hutchinson TA, et al. Renal function following infusion of radiologic contrast material. A prospective controlled study.
Archives of internal medicine 1985;145:87-9.
55. Heller CA, Knapp J, Halliday J, O'Connell D, Heller RF. Failure to demonstrate contrast nephrotoxicity. The Medical journal of Australia
1991;155:329-32.
56. Langner S, Stumpe S, Kirsch M, Petrik M, Hosten N. No increased risk for contrast-induced nephropathy after multiple CT perfusion studies of
the brain with a nonionic, dimeric, iso-osmolal contrast medium. AJNR. American journal of neuroradiology 2008;29:1525-9.
57. Lima FO, Lev MH, Levy RA, et al. Functional Contrast-Enhanced CT for Evaluation of Acute Ischemic Stroke Does Not Increase the Risk of
Contrast-Induced Nephropathy. American Journal of Neuroradiology 2010;31:817-21.
58. McGillicuddy EA, Schuster KM, Kaplan LJ, et al. Contrast-induced nephropathy in elderly trauma patients. The Journal of trauma 2010;68:294-
7.
59. Oleinik A, Romero JM, Schwab K, et al. CT angiography for intracerebral hemorrhage does not increase risk of acute nephropath y. Stroke
2009;40:2393-7.
60. Tremblay LN, Tien H, Hamilton P, et al. Risk and benefit of intravenous contrast in trauma patients with an elevated serum creatinine. The
Journal of trauma 2005;59:1162-6; discussion 66-7.
61. Media ACoDaC. ACR Manual on Contrast Media. https://www.acr.org/Clinical-Resources/Contrast-Manual. Accessed April 22, 2021.
62. Parfrey PS, Griffiths SM, Barrett BJ, et al. Contrast material-induced renal failure in patients with diabetes mellitus, renal insufficiency, or both.
A prospective controlled study. The New England journal of medicine 1989;320:143-9.
63. Abujudeh HH, Gee MS, Kaewlai R. In emergency situations, should serum creatinine be checked in all patients before performing second
contrast CT examinations within 24 hours? Journal of the American College of Radiology : JACR 2009;6:268-73.
64. Trivedi H, Foley WD. Contrast-induced nephropathy after a second contrast exposure. Renal failure 2010;32:796-801.
65. Stacul F, Bertolotto M, Thomsen HS, et al. Iodine-based contrast media, multiple myeloma and monoclonal gammopathies: literature review and
ESUR Contrast Media Safety Committee guidelines. European radiology 2018;28:683-91.
66. Pahade JK, LeBedis CA, Raptopoulos VD, et al. Incidence of contrast-induced nephropathy in patients with multiple myeloma undergoing
contrast-enhanced CT. AJR Am J Roentgenol 2011;196:1094-101.
67. Crowley MP, Prabhakaran VN, Gilligan OM. Incidence of Contrast-Induced Nephropathy in Patients with Multiple Myeloma Undergoing
Contrast-Enhanced Procedures. Pathology oncology research : POR 2018;24:915-19.
68. Kim SM, Cha RH, Lee JP, et al. Incidence and outcomes of contrast-induced nephropathy after computed tomography in patients with CKD: a
quality improvement report. American journal of kidney diseases : the official journal of the National Kidney Foundation 2010;55:1018-25.
69. Thomsen HS, Morcos SK. Risk of contrast-medium-induced nephropathy in high-risk patients undergoing MDCT--a pooled analysis of two
randomized trials. European radiology 2009;19:891-7.
70. Weisbord SD, Mor MK, Resnick AL, Hartwig KC, Palevsky PM, Fine MJ. Incidence and outcomes of contrast-induced AKI following computed
tomography. Clinical journal of the American Society of Nephrology : CJASN 2008;3:1274-81.
71. Nyman U, Ahlkvist J, Aspelin P, et al. Preventing contrast medium-induced acute kidney injury : Side-by-side comparison of Swedish-ESUR
guidelines. European radiology 2018;28:5384-95.
72. Too CW, Ng WY, Tan CC, Mahmood MI, Tay KH. Screening for impaired renal function in outpatients before iodinated contrast injection:
Comparing the Choyke questionnaire with a rapid point-of-care-test. European journal of radiology 2015;84:1227-31.
73. Choyke PL, Cady J, DePollar SL, Austin H. Determination of serum creatinine prior to iodinated contrast media: is it necessary in all patients?
Techniques in urology 1998;4:65-9.
74. Tippins RB, Torres WE, Baumgartner BR, Baumgarten DA. Are screening serum creatinine levels necessary prior to outpatient CT
examinations? Radiology 2000;216:481-4.
75. Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute renal failure - definition, outcome measures, animal models, fluid therapy and
information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Critical
care (London, England) 2004;8:R204-12.
76. Lakhal K, Ehrmann S, Chaari A, et al. Acute Kidney Injury Network definition of contrast-induced nephropathy in the critically ill: incidence and
outcome. Journal of critical care 2011;26:593-9.
77. Nash K, Hafeez A, Hou S. Hospital-acquired renal insufficiency. American journal of kidney diseases : the official journal of the National
Kidney Foundation 2002;39:930-6.
78. Valette X, Parienti JJ, Plaud B, Lehoux P, Samba D, Hanouz JL. Incidence, morbidity, and mortality of contrast -induced acute kidney injury in a
surgical intensive care unit: a prospective cohort study. Journal of critical care 2012;27:322.e1-5.
POST-CONTRAST ACUTE KIDNEY INJURY AND CONTRAST-INDUCED NEPHROPATHY IN ADULTS 43
79. Faucon AL, Bobrie G, Clément O. Nephrotoxicity of iodinated contrast media: From pathophysiology to prevention strategies. European journal
of radiology 2019;116:231-41.
80. Subramaniam RM, Wilson RF, Turban S, et al. AHRQ Comparative Effectiveness Reviews. Contrast-Induced Nephropathy: Comparative
Effectiveness of Preventive Measures. Rockville (MD): Agency for Healthcare Research and Quality (US); 2016.
81. Kooiman J, Seth M, Share D, Dixon S, Gurm HS. The association between contrast dose and renal complications post PCI across t he continuum
of procedural estimated risk. PloS one 2014;9:e90233.
82. Barrett BJ, Carlisle EJ. Metaanalysis of the relative nephrotoxicity of high- and low-osmolality iodinated contrast media. Radiology
1993;188:171-8.
83. Aspelin P, Aubry P, Fransson SG, Strasser R, Willenbrock R, Lundkvist J. Cost-effectiveness of iodixanol in patients at high risk of contrast-
induced nephropathy. American heart journal 2005;149:298-303.
84. Barrett BJ, Katzberg RW, Thomsen HS, et al. Contrast-induced nephropathy in patients with chronic kidney disease undergoing computed
tomography: a double-blind comparison of iodixanol and iopamidol. Investigative radiology 2006;41:815-21.
85. Feldkamp T, Baumgart D, Elsner M, et al. Nephrotoxicity of iso-osmolar versus low-osmolar contrast media is equal in low risk patients. Clinical
nephrology 2006;66:322-30.
86. Liss P, Persson PB, Hansell P, Lagerqvist B. Renal failure in 57 925 patients undergoing coronary procedures using iso-osmolar or low-osmolar
contrast media. Kidney international 2006;70:1811-7.
87. Heinrich MC, Häberle L, Müller V, Bautz W, Uder M. Nephrotoxicity of iso-osmolar iodixanol compared with nonionic low-osmolar contrast
media: meta-analysis of randomized controlled trials. Radiology 2009;250:68-86.
88. Barrett BJ, Parfrey PS. Clinical practice. Preventing nephropathy induced by contrast medium. The New England journal of medicine
2006;354:379-86.
89. Weisbord SD, Palevsky PM. Prevention of contrast-induced nephropathy with volume expansion. Clinical journal of the American Society of
Nephrology : CJASN 2008;3:273-80.
90. Merten GJ, Burgess WP, Gray LV, et al. Prevention of contrast-induced nephropathy with sodium bicarbonate: a randomized controlled trial.
Jama 2004;291:2328-34.
91. Navaneethan SD, Singh S, Appasamy S, Wing RE, Sehgal AR. Sodium bicarbonate therapy for prevention of contrast -induced nephropathy: a
systematic review and meta-analysis. American journal of kidney diseases : the official journal of the National Kidney Foundation 2009;53:617-
27.
92. Taylor AJ, Hotchkiss D, Morse RW, McCabe J. PREPARED: Preparation for Angiography in Renal Dysfunction: a randomized trial of inpatient
vs outpatient hydration protocols for cardiac catheterization in mild-to-moderate renal dysfunction. Chest 1998;114:1570-4.
93. Zoungas S, Ninomiya T, Huxley R, et al. Systematic review: sodium bicarbonate treatment regimens for the prevention of contrast-induced
nephropathy. Annals of internal medicine 2009;151:631-8.
94. Brar SS, Aharonian V, Mansukhani P, et al. Haemodynamic-guided fluid administration for the prevention of contrast-induced acute kidney
injury: the POSEIDON randomised controlled trial. Lancet (London, England) 2014;383:1814-23.
95. van der Molen AJ, Reimer P, Dekkers IA, et al. Post-contrast acute kidney injury. Part 2: risk stratification, role of hydration and other
prophylactic measures, patients taking metformin and chronic dialysis patients : Recommendations for updated ESUR Contrast Medium Safety
Committee guidelines. European radiology 2018;28:2856-69.
96. Solomon RJ, Natarajan MK, Doucet S, et al. Cardiac Angiography in Renally Impaired Patients (CARE) study: a randomized double-blind trial
of contrast-induced nephropathy in patients with chronic kidney disease. Circulation 2007;115:3189-96.
97. Nijssen EC, Nelemans PJ, Rennenberg RJ, Theunissen RA, van Ommen V, Wildberger JE. Prophylaxis in High -Risk Patients With eGFR < 30
mL/min/1.73 m2: Get the Balance Right. Investigative radiology 2019;54:580-88.
98. Nijssen EC, Rennenberg RJ, Nelemans PJ, et al. Prophylactic hydration to protect renal function from intravascular iodinated contrast material in
patients at high risk of contrast-induced nephropathy (AMACING): a prospective, randomised, phase 3, controlled, open-label, non-inferiority
trial. Lancet (London, England) 2017;389:1312-22.
99. Weisbord SD, Gallagher M, Jneid H, et al. Outcomes after Angiography with Sodium Bicarbonate and Acetylcysteine. The New England journal
of medicine 2018;378:603-14.
100. Solomon R, Werner C, Mann D, D'Elia J, Silva P. Effects of saline, mannitol, and furosemide on acute decreases in renal funct ion induced by
radiocontrast agents. The New England journal of medicine 1994;331:1416-20.
101. Pannu N, Wiebe N, Tonelli M. Prophylaxis strategies for contrast-induced nephropathy. Jama 2006;295:2765-79.
102. Kawashima S, Takano H, Iino Y, Takayama M, Takano T. Prophylactic hemodialysis does not prevent contrast-induced nephropathy after
cardiac catheterization in patients with chronic renal insufficiency. Circulation journal : official journal of the Japanese Circulation Society
2006;70:553-8.
103. Cruz DN, Goh CY, Marenzi G, Corradi V, Ronco C, Perazella MA. Renal replacement therapies for prevention of radiocontrast-induced
nephropathy: a systematic review. The American journal of medicine 2012;125:66-78.e3.
Revision History
July 2021: Updated to reflect the ACR-NKF Consensus Statement
19 November 2014: Major revisions
15 April 2013: Minor revisions
23 June 2010: Major revisions
29 October 2008: First version

METFORMIN 44
METFORMIN
Metformin is a biguanide oral anti-hyperglycemic agent used primarily, but not exclusively, to treat patients with non-
insulin-dependent diabetes mellitus [1-3]. It is available as a generic drug as well as in proprietary formulations, alone
and in combination with other drugs (see Table A for some of the brand name formulations). The drug was approved in
the United States in December of 1994 for use as monotherapy or combination therapy in patients with non-insulin-
dependent diabetes mellitus whose hyperglycemia is not controlled by diet or sulfonylurea therapy alone.
Metformin is thought to act by decreasing hepatic glucose production and enhancing peripheral glucose uptake as a
result of increased sensitivity of peripheral tissues to insulin. Only rarely does it cause hypoglycemia.
The most significant adverse effect of metformin therapy is the potential for the development of metformin -associated
lactic acidosis in susceptible patients. This condition is estimated to occur at a rate of 0 to 0.084 cases per 1,000 patient
years. Patient mortality in reported cases is about 50%. However, in almost all reported cases, lactic acidosis occurred
because one or more patient-associated contraindications for the drug were overlooked. In one extensive 13-year
retrospective study [4] of patients in Sweden, 16 cases were found, and all patients had several comorbid factors, most
often cardiovascular or renal disease. There are no documented cases of metformin-associated lactic acidosis in properly
selected patients.
Metformin is excreted unchanged by the kidneys, probably by both glomerular filtration and tubular excretion. The renal
route eliminates approximately 90% of the absorbed drug within the first 24 hours. Metformin seems to cause increased
lactic acid production by the intestines. Any factors that decrease metformin excretion or increase blood lactate levels are
important risk factors for lactic acidosis. Renal insufficiency, then, is a major consideration for radiologists.
Also, factors that depress the ability to metabolize lactate, such as liver dysfunction or alcohol abuse, or that increase
lactate production by increasing anaerobic metabolism (e.g., cardiac failure, cardiac or peripheral muscle ischemia, or
severe infection) are contraindications to the use of metformin. Iodinated X-ray contrast media are not an independent
risk factor for patients taking metformin but are a concern only if post-contrast acute kidney injury (AKI) should
develop. Please refer to the chapter on Postcontrast Acute Kidney Injury and Contrast-Induced Nephropathy in Adults
for information about the risk of these events.
The metformin package inserts approved by the U.S. Food and Drug Administration state that metformin should be
withheld temporarily for patients undergoing radiological studies using IV iodinated contrast media. If acute kidney
injury were to be caused by the iodinated contrast media, an accumulation of metformin could occur, with resultant
lactate accumulation.
Management
The management of patients taking metformin should be guided by the following:
1.
Patients taking metformin are not at higher risk than other patients for post-contrast acute kidney injury.
2.
Iodinated contrast is a potential concern for furthering renal damage in patients with acute kidney injury, and in
patients with severe chronic kidney disease (stage IV or stage V).
3.
There have been no reports of lactic acidosis following intravenous iodinated contrast medium administration in
patients properly selected for metformin administration.
The Committee recommends that patients taking metformin be classified into one of two categories based on the
patient’s renal function (as measured by eGFR).
Category I
In patients with no evidence of AKI and with eGFR ≥30 mL / min/1.73m
2
, there is no need to discontinue metformin
either prior to or following the intravenous administration of iodinated contrast media, nor is there an obligatory need to
METFORMIN 45
reassess the patient’s renal function following the test or procedure.
1
Category II
In patients taking metformin who are known to have acute kidney injury or severe chronic kidney disease (stage IV or
stage V; i.e., eGFR< 30), or are undergoing arterial catheter studies that might result in emboli (atheromatous or other) to
the renal arteries, metformin should be temporarily discontinued at the time of or prior to the procedure and withheld for
48 hours subsequent to the procedure and reinstituted only after renal function has been re-evaluated and found to be
normal.
Metformin and Gadolinium
It is not necessary to discontinue metformin prior to contrast medium administration when the amount of gadolinium-
based contrast material administered is in the usual dose range of 0.1 to 0.3 mmol per kg of body weight.
1The U.S. Food and Drug Administration (FDA) has issued guidelines and drug labeling for metformin since 1995, and the component of these
FDA guidelines related to administration of iodinated contrast material in patients taking metformin has been made progressiv ely less rigorous
since the original version. The ACR Committee on Drugs and Contrast Media recognizes that the latest (as of this writing, dat ed 4-8-2016) FDA
guidelines and drug labeling are still more restrictive than those in this chapter of the ACR Manual on Contrast Media. Nevertheless, the committee
authoring this Manual has reviewed the evidence and believes that the prevailing weight of clinical evidence on this matter allows less stringent yet
safe patient management which should reduce patient cost and inconvenience. This footnote is designed to alert readers that the ACR
recommendations differ in case their personal philosophy or institutional policies necessitate adherence to the more restrictive FDA guidelines.
Table A
Medications containing Metformin*
Generic Ingredients
Trade Names
Metformin
Glucophage
Glucophage XR
Fortamet
Glumetza
Riomet
Glyburide/metformin
Glucovance
Glipizide/metformin
Metaglip
Linigliptin.metformin
Jentadueto
Pioglitazone/metformin
ActoPlus Met
ActoPlus Met XR
Repaglinide/metformin
Prandimet
Rosiglitazone/metformin
Avandamet
Saxagliptin/metformin
Kombiglyze XR
Sitagliptin/metformin
Janumet
Janumet XR
(Metformin and several of the combination drugs also available in generic versions)
*List most recently revised on 4/17/2014
References
1.
Bailey CJ, Turner RC. Metformin. The New England journal of medicine. 1996;334(9):574-579.
2.
Dunn CJ, Peters DH. Metformin. A review of its pharmacological properties and therapeutic use in non-insulin-dependent diabetes
mellitus. Drugs. 1995;49(5):721-749.
3.
Sirtori CR, Pasik C. Re-evaluation of a biguanide, metformin: mechanism of action and tolerability. Pharmacological research: the official
journal of the Italian Pharmacological Society. 1994;30(3):187-228.
4.
Wiholm BE, Myrhed M. Metformin-associated lactic acidosis in Sweden 1977-1991. European journal of clinical pharmacology.
1993;44(6):589-591.
Revision History
12 December 2014: Minor revisions
METFORMIN 46
29 June 2012: Minor revisions
29 October 2008: First version

CONTRAST MEDIA IN CHILDREN 47
CONTRAST MEDIA IN CHILDREN
Principles regarding contrast media utilization and associated adverse events are generally similar in children and adults. This
section will address specific areas in which pediatric use of contrast medium differs from adult use and will attempt to avoid
repeating recommendations that are similar for both patient populations.
Iodinated Intravascular Contrast Media
Osmolality
Osmolality, a measure of the concentration of a solution, is an important physical property of contrast media. There is varia tion
in the osmolality of the nonionic iodinated contrast media approved for use in the United States, even when the iodine
concentration is equivalent (see Appendix A). Contrast media osmolality is of particular importance in neonates and small
children who are especially susceptible to fluid shifts and have a lower tolerance for intravascular and enteric osmotic loads
compared to adults. Intravascular administration of hyperosmolar contrast medium can result in the shift of fluid from the
extravascular space into the intravascular space, expanding blood volume [1,2]. If the fluid shift is large, cardiac failure and
pulmonary edema can result; children with significant pre-existing cardiac dysfunction may be at particular risk.
Viscosity
Viscosity, a measure of fluid resistance to stress, is another important physical property of contrast media and varies between
different contrast media (see Appendix A). Contrast medium viscosity is especially relevant in pediatric patients due to the use
of small gauge angiocatheters for contrast medium injection. Smaller gauge catheters and higher contrast medium viscosity both
slow the injection flow rate per Poiseuille’s law. If rapid injection of a high viscosity contrast medium through a small
angiocatheter is attempted, catheter failure, vessel injury, or failure to achieve the desired rapid injection rate may result.
Viscosity of contrast media is inversely affected by temperature (see Appendix A). As temperature increases, viscosity
decreases, thereby allowing for increased flow rates at lower pressures. However, there is a lack of data demonstrating the
benefits of contrast media warming in children [3,4].
Safe Intravascular Injection of Contrast Media in Children
Several additional issues complicate the administration of intravascular contrast media to neonates and children, including the
use of small volumes of contrast medium, small gauge angiocatheters, and unusual vascular access sites. First, very small
volumes of contrast media are typically administered to neonates and infants (typically 1.5–2 mL/kg) [5]. As a result, timing of
image acquisition with regard to contrast medium administration may be important when performing certain imaging studies,
such as CT angiography. In some instances, a slower injection rate (compared to that used in older children and adults) may be
useful to prolong intravascular enhancement. Second, small-gauge angiocatheters (e.g., 24-gauge) located in very small
peripheral veins (e.g., in the hand or foot) or other unusual vascular access sites are commonly utilized in neonates and infants.
A study by Amaral et al [6] showed that 24-gauge angiocatheters in a peripheral location can be safely power injected using a
maximum flow rate of approximately 1.5 mL/sec and a maximum pressure of 150 psi. Recently, new small gauge intravenous
catheter systems (e.g., BD Nexiva™ Diffusics™ closed IV catheter system) have been developed that allow injection rates and
pressures higher than traditional peripheral intravenous cannulas, although there is a paucity of data describing their use in
children undergoing power injection of contrast medium. When access is thought to be tenuous, careful hand injection of
contrast medium should be strongly considered to minimize risk of vessel injury and extravasation, noting that smaller syringes
can generate substantially greater injection pressures than larger syringes. Since some central venous catheters may not be
approved for power injection, one should always verify in advance that any catheter to be utilized for bolus contrast material
instillation can tolerate the anticipated injection and is appropriately positioned. It is also important to ensure that the pressure
used does not exceed the catheter’s pressure rating.
Extravasation of Contrast Media in Children
The frequency of contrast media extravasation in children is likely similar to that observed in the adult population, despite the
use of lower intravascular contrast medium volumes. An extravasation rate of 0.3% was documented in a study of 554 children
in which a power injector was used to administer iodinated contrast medium [6]. Another study of 2429 children who
underwent intravenous power injection of iodinated contrast medium found an extravasation rate of 0.7% without an association
with age [7]. Most extravasations in the pediatric population resolve without complication. A study by Wang et al [8] showed
that 15 of 17 cases of contrast medium extravasation in children were mild in severity with minimal or no adverse effects. In the
study by Barrera et al [7], none of the 18 children who experienced a contrast extravasation suffered a long-term substantial

CONTRAST MEDIA IN CHILDREN 48
injury or required surgery, although moderate and severe cases were documented. Furthermore, this study demonstrated positive
associations between contrast media extravasation and both power injection peak pressure and flow rate.
Particular attention should be paid to the injection sites of neonates, infants, young children and developmentally delayed
patients, as they cannot verbalize symptoms of an injection site complication. Please refer to the Extravasation of Contrast
Media Chapter or download the ACR Guidance – Contrast Reaction App for the treatment of a contrast extravasation, as
treatment in a child is similar to that in an adult.
Physiologic Side Effects in Children
Physiologic side effects to intravascular contrast medium administration in children have the potential for greater consequence
than in adults [9]. For example, local warmth at the injection site or nausea may cause a child to move or cry. Such a response
to contrast medium injection may result in a nondiagnostic imaging study, necessitating repeat imaging and additional exposure
to contrast medium and possibly ionizing radiation. Management of physiologic side effects is generally supportive.
Incidence of Allergic-Like Reactions
There are several difficulties in interpreting the available literature on the incidence of allergic-like reactions to IV iodinated
contrast media in children. First, some studies have failed to discriminate between physiologic side effects and allergic-like
reactions and have used heterogeneous definitions of what constitutes mild, moderate, or severe reactions. Second, there is a
lack of controlled prospective pediatric studies on the topic. Prospective investigations are difficult to perform because allergic-
like reactions to contrast media in children are rare, and large numbers of patients would be needed to acquire statistically
meaningful results. Third, in some studies in the published literature, the methodology for tracking contrast reactions is such
that it is difficult to guarantee that all adverse reactions were documented, and none were overlooked.
Therefore, the reported incidence of pediatric allergic-like reactions to contrast media is variable. It is generally agreed,
however, that the incidence of allergic-like reactions in children is lower than that in adults [3,8-10]. A very large retrospective
study by Katayama et al of more than 150,000 nonionic contrast medium administrations [10], when stratified by age, showed
that patients less than 10 years of age and greater than 60 years of age have the lowest rates of adverse reactions overall; the rate
of severe reactions was more uniform across age groups but the numbers were small. A study by Dillman et al [11]
retrospectively reviewed more than 11,000 intravascular injections of low-osmolality nonionic iodinated contrast media in
children and neonates and documented an allergic-like reaction rate of 0.18%. Of the 20 reactions documented in their study, 16
were mild, one was moderate, and three were severe. A study by Callahan et al [12] of 12,494 consecutive patients up to 21
years of age revealed a 0.46% incidence of adverse reactions to ioversol, the majority of which were mild and none of which
were severe. A smaller study by Fjelldal et al [13] documented five allergic-like reactions to iohexol following a total of 547
injections, for a rate of reaction of 0.9%. Although fatal reactions to contrast media in children are extremely rare (and may be
due to co-morbid conditions), infants, young children, and developmentally delayed patients require close observation during
and immediately following intravascular contrast medium administration, as they are unable to verbalize reaction-related
discomfort or symptoms.
Prevention of Allergic-Like Reactions
General guidelines for the prevention of allergic-like reactions in children are similar to those used for adult patients. However,
it should be noted that there has been no prospective, controlled investigation performed to assess the efficacy of premedic ation
for the prevention of allergic-like reactions to iodinated contrast media in children. A sample pediatric premedication regimen,
using a combination of corticosteroid and antihistamine, is described in Table A at the end of this chapter. Allergic-like
reactions following premedication may still occur, although the frequency of such reactions is unknown [11].
Treatment of Allergic-Like Reactions
General guidelines for the treatment of allergic-like reactions in children are similar to those used for adult patients. Pediatric
medication dosages, however, may be significantly different from adult dosages used in the management of such reactions
(Table 2 and Table 3). It can be helpful to have a pediatric medication chart with weight-based dosages placed on the
emergency cart or posted in the rooms where intravascular contrast media is to be injected into children, noting that for IM
epinephrine administration, most pediatric patients can be treated with an autoinjector (i.e., EpiPen® or EpiPen Jr®) to
maximize speed of administration and minimize dosing errors. Dedicated pediatric emergency resuscitation equipment
(including various sizes of supplemental oxygen facemasks) should be available in all such locations (Table 4). A separate box
of pediatric airway equipment attached to the emergency cart may be useful in areas where both children and adults receive
contrast media.

CONTRAST MEDIA IN CHILDREN 49
Acute Kidney Injury after Iodinated Contrast Media Administration in Children
Acute kidney injury (AKI) after contrast media administration is subdivided into two entities per the ACR-NKF consensus
statement: contrast-induced acute kidney injury (CI-AKI), which implies a causal relationship between iodinated contrast
material and AKI, and contrast-associated acute kidney injury (CA-AKI) [also referred to as post-contrast acute kidney injury
(PC-AKI)], which represents a correlative relationship between iodinated contrast material (ICM) and acute kidney injury [14].
Recent studies in which children exposed to intravenous iodinated contrast media were propensity score matched with groups of
nonexposed control patients have showed no significant difference in the frequencies of subsequent acute kidney injury (CI -
and CA-AKI) [15,16]. A study by Gilligan et al of 925 ICM-exposed children 18 years of age and younger propensity score
matched to a group who underwent abdominal ultrasound showed no significant difference in the frequency of CI-AKI between
the exposed and unexposed groups and found that, in children with an eGFR of 60 and greater, iodinated contrast medium
exposure was not predictive of CI-AKI [15]. A similar large propensity score-based study by Calle-Toro et al. yielded similar
findings [17]. A study by Guo et al of 192 ICM-exposed children 3-years-old or younger prior to cardiac surgery propensity
score matched to a group without preoperative iodinated contrast media exposure found no significant difference in
postoperative AKI [16]. A retrospective study of 211 trauma patients aged 15 years or younger (164 exposed to contrast, 47 not
exposed to contrast) showed no significant difference in frequency of subsequent AKI [18]. The effects of contrast media on the
kidneys are generally assumed to be similar between children and adults as no evidence currently available suggests otherwise.
A few key differences between CI-AKI and CA-AKI in children and adults are discussed below.
Measurement of Renal Function in Children
Serum creatinine concentration reflects the balance between creatinine production and excretion. Creatinine is a breakdown
product of skeletal muscle, and its rate of production is proportional to muscle mass. Muscle mass depends on a variety of
factors, including patient age, gender, and level of physical activity. Normal serum creatinine concentrations, thus, are quite
variable in pediatric patients. Normal pediatric serum creatinine concentrations increase with age, with the upper limits of
normal typically less than adult values. Age-based normal serum creatinine concentrations also may vary slightly from
laboratory to laboratory.
There are problems with using serum creatinine concentration as the sole marker of renal function. First, a normal serum
creatinine value does not mean that renal function is preserved. For example, an increase in creatinine from 0.4 mg/dL to 0.8
mg/dL in a 10-year-old child would be clinically important and suggest some degree of renal impairment, even though both
measurements may be within acceptable limits for patient age. Serum creatinine concentration may not become abnormal until
glomerular filtration has decreased substantially. Second, it may take several days in the setting of acute renal failure for serum
creatinine concentration to rise. Therefore, a patient may have impaired renal function in the setting of a normal serum
creatinine concentration. This phenomenon is not unique to the pediatric population.
Measurement of blood urea nitrogen (BUN) concentration is a poor indicator of renal function. BUN concentration depends on
numerous variables in addition to renal function, including daily dietary protein intake, hepatic function, and patient hydration.
A more appropriate method by which to express renal function in children is the estimated glomerular filtration rate (eGFR). It
is important to note that the formula used to calculate pediatric eGFR (see below) is different from that used in adults. eGFR
calculation in children requires knowledge of patient serum creatinine concentration, height, and/or cystatin C. In addition, the
assay used to measure serum creatinine concentration must be known.
EGFR Calculation in Children
There is no perfect manner of estimating GFR in children. The National Kidney Disease Education Program, an initiative of the
National Institutes of Health, provides an online calculator for estimating purposes and has published the following information
regarding the estimation of GFR in children.
Currently, the best equation for estimating GFR from serum creatinine in children and young adults ages 1-25 years is the CKiD
Under 25 (U25) eGFR [19]. This formula has improved validity across a broader age range compared to the previously
recommended Bedside Schwartz Equation, which was developed using a narrow age range of 8-15 years old. The CKiD U25
eGFR equation can be used with the serum creatinine level or with the IFCC-calibrated serum cystatin C level; both require age,
sex, and height.

CONTRAST MEDIA IN CHILDREN 50
Equation: CKiD U25 eGFR
eGFR (mL/min/1.73 m2) = (K × height) / serum creatinine AND/OR
eGFR (mL/min/1.73 m2) = K x (1/cystatin C)
•
K is multiplier that is dependent on age and sex
•
Height in cm
•
Serum creatinine in mg/dL
•
Cystatin C in mg/L
It is also important to note that in the immediate newborn period (approximately the first 3 weeks of life), blood creatinine
concentration represents at least in part the maternal creatinine concentration which freely crosses the placenta [20]. Thus,
infant plasma creatinine is not a reliable measure of renal function or glomerular filtration rate. Iodinated contrast media can be
generally considered safe in the neonate and infant regardless of serum creatinine and eGFR in the absence of known kidney
disease [21].
Prevention of Contrast-Induced and Contrast-Associated Acute Kidney Injury in At-Risk Children
Risk factors for CI-AKI and CA-AKI in children are thought to be similar to those in adults. Unfortunately, there are no
established evidence-based guidelines for the prevention of CI-AKI and CA-AKI in children with impaired renal function. As
no pediatric-specific measures for the prevention of acute kidney injury have been established in the literature, strategies
described in adults should be considered when using IV iodinated contrast media in children with renal dysfunction. Please
refer to the chapter Post-Contrast Acute Kidney Injury And Contrast-Induced Nephropathy In Adults. A noncontrast imaging
examination can be performed if the clinical question can be answered without intravascular iodinated contrast medium. In
addition, the use of alternative imaging modalities, such as ultrasound (with or without intravenous microbubble contrast
medium) and MRI (with or without intravenous gadolinium-based contrast medium) could also be considered.
Thyroid Dysfunction after Iodinated Contrast in Children
Some published data suggests an increased risk of thyroid dysfunction after the administration of iodinated contrast media in
particular pediatric cohorts, including infants with congenital heart disease and very low birth weight. Supraphysiologic levels
of iodine can potentially result in suppression of thyroid hormone production, a phenomenon known as the Wolff-Chaikoff
effect (a protective autoregulatory mechanism). This effect in most individuals is transient, and thyroid hormone production
typically returns to normal after 1-2 weeks. However, it is thought that in particularly susceptible populations, some individuals
are unable to escape the Wolff-Chaikoff effect, resulting in true hypothyroidism.
The United States Food and Drug Administration (FDA) issued a safety announcement in 2015 regarding 10 cases of
hypothyroidism reported after ICM administration in infants less than 4 months of age that occurred between 1969 and 2012
[22]. Each of those infants had congenital heart disease or a history of prematurity.
There is a small number of prospective studies that have evaluated the association between thyroid dysfunction and
intravascular iodinated contrast media exposure in children. These studies have shown mixed results and are generally very
small with regards to the number of participants, may or may not have a control group, and study specific pediatric cohorts
which limit the generalizability of observations. There are some retrospective studies with larger (e.g., n >100) patient
populations that also have assessed the impact of intravascular iodinated contrast media on thyroid function. These latter studies
are also mostly limited due to a lack of control groups and/or a variety of biases and confounders. A review of this literature can
be found in the ACR Statement on Use of Iodinated Contrast Material for Medical Imaging in Young Children and Need for
Thyroid Monitoring [23].
In summary, there is an overall lack of high-quality data available regarding the risk of thyroid dysfunction after intravascular
iodinated contrast media exposure and further investigation is needed.
Thyroid Monitoring after Iodinated Contrast in Children
The FDA issued an updated Drug Safety Communication in March 2022 recommending that newborns and children through 3
years of age undergo thyroid monitoring within 3 weeks after receiving intravascular iodinated contrast media [24]. This
announcement cited 11 studies, nearly all of which have considerable limitations, as detailed in the section above. The ACR’s
position statement on this topic, referred to above, indicates that based on the available evidence the risk for clinically relevant
hypothyroidism related to iodinated contrast media is quite low in children younger than 3 months and minimal to absent in

CONTRAST MEDIA IN CHILDREN 51
children 3 months and older. Furthermore, the ACR statement suggests that universal testing in all young children is not
currently supported by the published literature [23].
Gadolinium-Based Intravascular Contrast Media
Guidelines for intravenous (IV) use of gadolinium-based contrast media are generally similar in both the pediatric and adult
populations. The prescribing information sheets from gadolinium-based contrast media approved for intravascular use in the
United States show variable labeling with regards to the pediatric population, with differences between contrast media relating
to indications (most often MRI of the brain and spine, and less often other parts of the body) and age of approval for usage. For
example, for certain contrast media, safety and efficacy have been established for pediatric patients over 2 years of age but not
for children under 2 years of age. No gadolinium-based contrast media has been approved for use in premature infants to date.
The prescribing information for gadoxetate disodium (Eovist), a hepatobiliary contrast media, does not specifically specify
patient age for which the drug is approved. Instead, it indicates that an observational study was performed in patients aged >2
months and <18 years and identified no safety issues. Based on the above information, it can be concluded that these contrast
media are commonly used off-label in children in the U.S. with regards to patient age, clinical indication for imaging, or both.
Pediatric-specific issues regarding these contrast media are discussed below.
Osmolality and Viscosity
As with iodinated contrast media, there are significant ranges in osmolality and viscosity of gadolinium- based contrast media
(see Appendix A). For example, the osmolality of gadoteridol (ProHance) is 630 mosm/kg H2O, and the osmolality of
gadobenate dimeglumine (MultiHance) is 1,970 mosm/kg H2O. Viscosities (at 37°C) can also vary, for example from 1.19 cps
for gadoxetate disodium (Eovist) to 5.3 cps for gadobenate dimeglumine (MultiHance). These physical properties, however,
potentially are less important when using gadolinium-based contrast media in children compared to iodinated contrast agents.
The much smaller volumes of gadolinium-based contrast agents typically administered to pediatric patients likely result in only
minimal fluid shifts. The slower injection flow rates generally used for gadolinium-based contrast result in lower injection-
related pressures and decreased risk for vessel injury and extravasation. While there is a lack of published literature describing
the frequency and clinical relevance of extravasation of these contrast media in children, it is usually of no or minimal clinical
significance because of the small volumes injected.
Allergic-Like Reactions and Other Adverse Events
Although uncommon, allergic-like reactions to IV gadolinium-based contrast media in children do occur. A study by Dillman et
al [25] documented a 0.04% (48 reactions/13,344 injections) allergic-like reaction rate for these contrast media in children.
Another study by Davenport et al that included 15,706 administrations of gadolinium-based contrast media in children (under
the age of 18 years) documented only eight allergic-like reactions, for a reaction rate of 0.05% [26]. A more recent study by
Forbes-Amrhein et al. documented 21 allergic-like reactions in 32,365 pediatric gadolinium-based contrast media
administrations [27]. Although mild reactions are most common, more significant reactions that require urgent medical
management may occur [27]. Pediatric allergic-like reactions to contrast media are treated in the same manner as other allergic
and allergic-like reactions, including similar reactions to iodinated contrast media (Table 2). While no investigation has studied
the efficacy of corticosteroid and antihistamine premedication regimens for the prevention of allergic-like reactions to
gadolinium-based contrast media in children, commonly used regimens, such as the ones presented in Tables A and B at the end
of the chapter, are thought to provide some protective benefit.
A variety of physiologic side effects also may occur following administration of gadolinium-based contrast media, including
coldness at the injection site, nausea, vomiting, headache, and dizziness (see prescribing information for different contrast
media). There is no evidence for pediatric renal toxicity from gadolinium-based contrast media at approved doses, and there is
no role for intravenous or oral hydration prior to IV administration of gadolinium-based contrast media.
Nephrogenic Systemic Fibrosis
There are only a small number of reported cases of nephrogenic systemic fibrosis (NSF) in children. As of September 2012,
there were only 23 unique pediatric NSF cases, and all patients were 6 years of age or older [28]. Seventeen of these childre n
had documented exposure to a gadolinium-based contrast media. Thirteen of 13 children with available clinical data pertaining
to renal disease had substantial renal dysfunction (acute kidney injury and/or chronic kidney disease), and 10 were on
hemodialysis and/or peritoneal dialysis at the time of gadolinium-based contrast medium administration. Renal status was
unknown in 10 children. There have been no published reports of NSF since 2012 in the pediatric population using currently
approved contrast media.
CONTRAST MEDIA IN CHILDREN 52
As there are no pediatric-specific evidence-based guidelines for the prevention of NSF, we recommend that adult guidelines be
followed for identifying at-risk patients and administering gadolinium-based contrast media in the presence of impaired renal
function [see Chapter 16 –Nephrogenic Systemic Fibrosis]. If use of a Group I or III GBCA is being considered, children at risk
for renal dysfunction should be identified (e.g., those with known medical renal disease [chronic kidney disease or acute kidney
injury] or those with known renal/urinary tract structural abnormalities) and screened for impaired renal function. As in adults,
the risks and benefits of gadolinium-based contrast media administration should be carefully considered in pediatric patients
with acute kidney injury as well as chronic kidney disease with an eGFR less than 30 mL/min/1.73 m2. Although there has been
no reported case of NSF in a very young child to date, some authors have urged caution with regards to administering these
contrast media to preterm neonates and infants [29] due to renal immaturity and potential glomerular filtration rates under 30
mL/min/1.73m2 [30]. However, as mentioned above, there have been no cases of NSF in neonates and infants, and these
contrast media may be administered to these populations if clinically warranted. Similar to older pediatric patients both without
and with impaired renal function, the use of IV gadolinium-based contrast media should be justified in very young children, and
the benefit of administration should outweigh potential risks.
Gadolinium Retention in the Body
Like adults, retention of gadolinium in the body has been observed by both imaging and/or pathology in children in multiple
tissues (including the brain) and with a variety of gadolinium-based contrast media, including macrocyclic media [31-33]. The
literature is conflicting and attributes these apparent signal alterations in the brain to a variety of causes including a particular
contrast media, previous brain irradiation, and/or patient age [34-39]. Also, similar to adults, we believe that IV gadolinium-
based contrast media can provide crucial diagnostic medical information. To date, no adverse health effects related to
gadolinium retention have been confirmed in the pediatric population. Despite this fact, each time a contrast-enhanced MRI
study is considered, it remains prudent to consider the clinical benefit of the diagnostic information that will be obtained while
avoiding overutilization. Retention is of particular concern in children with chronic medical conditions that may receive
multiple contrast-enhanced MRI studies over the course of their lifetimes. Reduced dose (e.g., half dose) contrast-enhanced
MRI has been described as a method to further reduce contrast media exposure in children [40], although further investigations
are needed before this practice can be commonplace. We will continue to assess the safety of these contrast media and modify
our recommendations accordingly as new data become available. See Chapter 14 – ACR-ASNR Position Statement on the Use
of Gadolinium Contrast Agents for additional information.
Gastrointestinal and Urogenital Contrast Media
Contrast media can be introduced into the gastrointestinal or urogenital tracts by mouth, rectum, vagina, urethra, urinary
bladder, ostomy, or indwelling gastrointestinal or urinary tract catheter. The most commonly used contrast media for
gastroenteric indications in infants, children, and adolescents are barium-based or water-soluble iodinated contrast media [41]
(Appendix A). Although iohexol is the only iodinated low osmolar contrast medium that is approved by the FDA for
gastrointestinal administration, any iodinated contrast medium that is FDA-approved for intravascular use is safe to administer
off-label into the gastrointestinal or urinary tract. Contrast media for urogenital imaging is typically iodine-based, although the
use of ultrasound microbubble contrast medium is becoming increasingly more common for pediatric studies involving the
urogenital tract.
Barium sulfate suspension is used in the assessment of the upper gastrointestinal tract and small bowel when there is no clinical
concern for perforation or leak. Barium sulfate is insoluble in water and absorption by the gut into the body is negligible.
Barium has a high atomic number (Z=56) and attenuates more x-rays than water-soluble contrast, making it preferred for fine
detail evaluation of the gastrointestinal system. Patients should be advised to stay well hydrated following an imaging study
with barium due to its water insolubility and tendency to constipate. Barium should not be administered retrograde into a
defunctionalized colon or bowel segment (e.g., mucous fistula or Hartman pouch), since intraluminal contents will likely not
pass and barium can become inspissated and difficult to remove. Barium is generally contraindicated in patients with suspected
or known gastrointestinal tract perforation because of the risk of peritonitis [42]. Barium contrast material is not directly toxic to
the airways; however, it does have the potential to plug distal airways, resulting in post-obstructive pneumonia or diminishing
the capacity for gas exchange [43]. Aspiration of large volumes of barium-based contrast can be fatal [44]. Allergic-like
reactions to barium sulfate preparations are extremely rare and are likely related to the presence of additives to the commercial
preparations rather than the barium sulfate [45]. Treatment of pediatric allergic-like reactions to enteric administration of
barium sulfate suspension is the same as for allergic-like reactions to intravascular iodine-based and gadolinium-based contrast
media and is outlined in Table 2.
Iodinated contrast media are commonly used for infant, pediatric, and adolescent contrast enemas, postoperative gastrointestinal
tract assessment, when there is concern for gastrointestinal tract perforation, and upper gastrointestinal tract studies in

CONTRAST MEDIA IN CHILDREN 53
premature and very-low-birthweight neonates. They are generally preferred in the setting of suspected gastrointestinal tract
perforation because they are quickly resorbed through the peritoneal surface and have a low risk of inducing peritonitis. The
exact iodinated contrast media used for a particular exam typically relates to a combination of availability, cost, iodine
concentration, and safety profile related to the route of administration.
As with intravascular iodinated contrast media, osmolality should be considered when deciding which iodinated contrast media
to administer via the gastrointestinal tract. Hyperosmolar, typically ionic iodinated contrast media such as diatrizoate
meglumine (i.e., Gastrografin or Gastroview) within the gastrointestinal tract may result in dangerous fluid shifts into the bowel
from the intravascular space and therefore are capable of causing dehydration and hypotension [46-50]. Neonates, infants of
very low birthweight, and older children with cardiac and renal impairment may be most susceptible to such fluid shifts. In such
patients, low-osmolality or iso-osmolality contrast media should be considered for imaging of the gastrointestinal tract. Higher
osmolality water soluble contrast media can be administered into the upper gastrointestinal tract or per rectum to assist in the
resolution of a bowel obstruction, such as in the setting of cystic fibrosis [51], adhesive bowel disease, or meconium ileus [52].
Use of such contrast media is also commonly carried out only after some degree of dilution [53-55]. However, per mouth
administration of high-osmolality iodinated contrast media should be generally avoided in children, in part due to the potential
consequences of aspiration. This risk can be mitigated by using of a nasogastric tube to deliver the contrast material into the
stomach. Aspirated hyperosmolality contrast medium may cause fluid shifts at the alveolar level and chemical pneumonitis with
resultant pulmonary edema [44,56-58]. Aspiration of large volumes of iodinated oral contrast media rarely may be fatal [44].
The taste of barium sulfate preparations is usually tolerated quite well by children, given the presence of flavoring and sugar
substitutes in the formulation. Barium sulfate suspensions also lend themselves well to flavor-mix-ins, such as chocolate syrup,
fruit syrups, or drink mix powders. The taste of some iodinated contrast media are bitter and can be a limitation in its oral
acceptability in pediatric patients. In this circumstance, placement of an enteric tube for contrast material administration can be
useful.
Urogenital contrast media typically include ionic iodine-based contrast media and ultrasound microbubble contrast media. The
iodinated contrast media are utilized in the setting of fluoroscopy and CT, whereas the ultrasound microbubbles are utilized in
the setting of contrast-enhanced voiding ultrasonography. Lumason® is the only ultrasound contrast media approved for
pediatric urinary tract use (see Ultrasound Contrast Media chapter). As with the gastrointestinal tract, any iodinated contrast
medium that is FDA-approved for intravascular use is safe to administer into the vagina, urethra, urinary bladder, or renal
collecting system.
Neutral oral contrast media are used for cross-sectional enterography imaging to promote distension of the bowel. These media
demonstrate water or near water attenuation on CT and biphasic signal intensity on MRI. Commercially available preparations
with imaging indications include a low-density barium sulfate suspension, which also contains sorbitol and a thickening agent
(NeuLumEX [formerly VoLumen]; Bracco Diagnostics, Milan, Italy) and a lemon-lime flavored beverage containing sorbitol,
mannitol, and a thickening agent (Breeza; Beckley Medical, Bristol, Conn). A study by Dillman et al showed that both media
provide adequate bowel distension for imaging, but pediatric patients are more likely to consume the entirety of their prescribed
oral preparation of Breeza versus NeuLumEX (VoLumen) due to the preferred flavor profile [59]. Other oral contrast media
options for cross sectional enterography include water, methylcellulose, and polyethylene glycol electrolyte solution (Golytely;
Braintree Laboratories, Inc, Braintree, Mass), each having unique advantages and disadvantages. Of these latter options,
polyethylene glycol has been demonstrated to have bowel distension similar to low-density barium suspension [60].
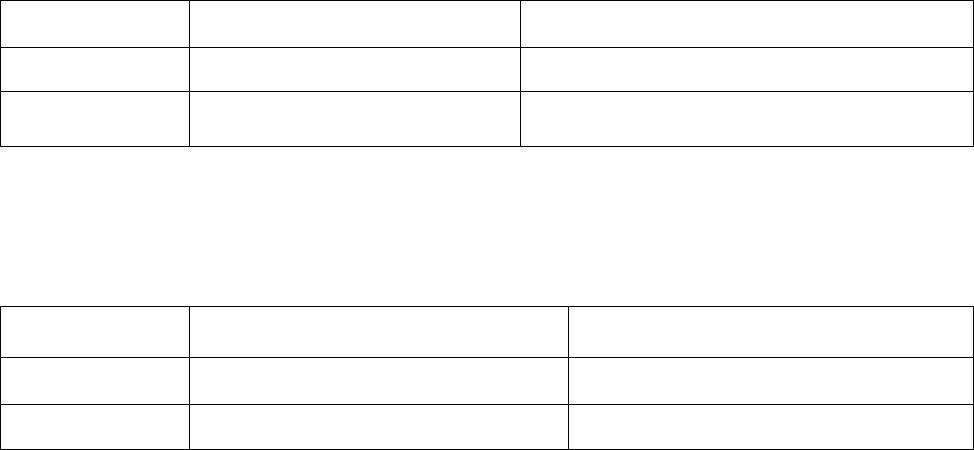
CONTRAST MEDIA IN CHILDREN 54
Table A
Sample Pediatric Corticosteroid and Antihistamine Premedication Regimen
Dosage
Timing
Prednisone
0.5–0.7 mg/kg PO (up to 50 mg)
13, 7, and 1 hr prior to contrast injection
Diphenhydramine
1.25 mg/kg PO (up to 50 mg)
1 hr prior to contrast injection
Note: Appropriate intravenous doses may be substituted for patients who cannot ingest PO medication.
Table B
Sample Pediatric Corticosteroid and Antihistamine Premedication Regimen for Urgent,
ED, Inpatient, or NPO Patients
Dosage
Timing
Hydrocortisone
2 mg/kg IV (up to 200 mg)
5 and 1 hr prior to contrast injection
Diphenhydramine
1 mg/kg IV, IM, or PO (up to 50 mg)
1 hr prior to contrast injection
Revision History
July 2024: Major Revision
2 June 2014: Minor revision
15 April 2013: Minor revision
29 June 2012: Minor revision
29 October 2008: First version
REFERENCES
1.
Standen JR, Nogrady MB, Dunbar JS, Goldbloom RB. The Osmotic Effects of Methylglucamine Diatrizoate (Renografin 60) in Intravenous
Urography in Infants. Am J Roentgenol Radium Ther Nucl Med 1965;93:473-9.
2.
Morris TW, Harnish PP, Reece K, Katzberg RW. Tissue fluid shifts during renal arteriography with conventional and low osmolal ity agents.
Invest Radiol 1983;18:335-40.
3.
Vergara M, Seguel S. Adverse reactions to contrast media in CT: effects of temperature and ionic property. Radiology 1996;199:363-6.
4.
Davenport MS, Wang CL, Bashir MR, Neville AM, Paulson EK. Rate of contrast material extravasations and allergic -like reactions: effect of
extrinsic warming of low-osmolality iodinated CT contrast material to 37 degrees C. Radiology 2012;262:475-84.
5.
Trout AT, Dillman JR, Ellis JH, Cohan RH, Strouse PJ. Patterns of intravenous contrast material use and corticosteroid premed ication in
children--a survey of Society of Chairs of Radiology in Children's Hospitals (SCORCH) member institutions. Pediatr Radiol 2011;41:1272-83.
6.
Amaral JG, Traubici J, BenDavid G, Reintamm G, Daneman A. Safety of power injector use in children as measured by incidence of
extravasation. AJR Am J Roentgenol 2006;187:580-3.
7.
Barrera CA, White AM, Shepherd AM, et al. Contrast Extravasation using Power Injectors for Contrast -Enhanced Computed Tomography in
Children: Frequency and Injury Severity. Acad Radiol 2019;26:1668-74.
8.
Wang CL, Cohan RH, Ellis JH, Adusumilli S, Dunnick NR. Frequency, management, and outcome of extravasation of nonionic iodinated
contrast medium in 69,657 intravenous injections. Radiology 2007;243:80-7.
9.
Cohen MD, Herman E, Herron D, White SJ, Smith JA. Comparison of intravenous contrast agents for CT studies in children. Acta Radiol
1992;33:592-5.
10.
Katayama H, Yamaguchi K, Kozuka T, Takashima T, Seez P, Matsuura K. Adverse reactions to ionic and nonionic contrast media. A report from
the Japanese Committee on the Safety of Contrast Media. Radiology 1990;175:621-8.
11.
Dillman JR, Strouse PJ, Ellis JH, Cohan RH, Jan SC. Incidence and severity of acute allergic-like reactions to i.v. nonionic iodinated contrast
material in children. AJR Am J Roentgenol 2007;188:1643-7.
12.
Callahan MJ, Poznauskis L, Zurakowski D, Taylor GA. Nonionic iodinated intravenous contrast material-related reactions: incidence in large
urban children's hospital--retrospective analysis of data in 12,494 patients. Radiology 2009;250:674-81.
13.
Fjelldal A, Nordshus T, Eriksson J. Experiences with iohexol (Omnipaque) at urography. Pediatr Radiol 1987;17:491-2.
14.
Davenport MS, Perazella MA, Yee J, et al. Use of Intravenous Iodinated Contrast Media in Patients With Kidney Disease: Consen sus Statements
from the American College of Radiology and the National Kidney Foundation. Kidney Med 2020;2:85-93.
15.
Gilligan LA, Davenport MS, Trout AT, et al. Risk of Acute Kidney Injury Following Contrast-enhanced CT in Hospitalized Pediatric Patients: A
Propensity Score Analysis. Radiology 2020;294:548-56.
16.
Guo S, Bai L, Tong Y, et al. Contrast media exposure in the perioperative period confers no additional risk of acute kidney injury in infants and
young children undergoing cardiac surgery with cardiopulmonary bypass. Pediatr Nephrol 2021;36:2485-91.
17.
Calle-Toro J, Viteri B, Ballester L, et al. Risk of Acute Kidney Injury Following Contrast-enhanced CT in a Cohort of 10 407 Children and
Adolescents. Radiology 2022:210816.
CONTRAST MEDIA IN CHILDREN 55
18.
Paul KM, 2nd, Johnson J, Garwe T, et al. Computed Tomography with Intravenous Contrast Is Not Associated with Development of Acute
Kidney Injury in Severely Injured Pediatric Patients. Am Surg 2019;85:e1-e5.
19.
Pierce CB, Muñoz A, Ng DK, Warady BA, Furth SL, Schwartz GJ. Age- and sex-dependent clinical equations to estimate glomerular filtration
rates in children and young adults with chronic kidney disease. Kidney Int 2021;99:948-56.
20.
Guignard JP, Drukker A. Why do newborn infants have a high plasma creatinine? Pediatrics 1999;103:e49.
21.
Bedoya MA, White AM, Edgar JC, Pradhan M, Raab EL, Meyer JS. Effect of Intravenous Administration of Contrast Media on Serum
Creatinine Levels in Neonates. Radiology 2017;284:530-40.
22.
US Food and Drug Administration. FDA Drug Safety Communication: FDA advises of rare cases of underactive thyroid in infants given iodine-
containing contrast agents for medical imaging. Available at: https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-
communication-fda-advises-rare-cases-underactive-thyroid-infants-given-
iodine#:~:text=11%2D17%2D2015%20Safety%20Announcement,and%20other%20medical%20imaging%20procedures. Accessed May, 2024.
23.
Dillman JR, Forbes-Amrhein MM, Wang CL, et al. ACR Statement on Use of Iodinated Contrast Material for Medical Imaging in Young
Children and Need for Thyroid Monitoring. J Am Coll Radiol 2022;19:849-53.
24.
US Food and Drug Administration. FDA recommends thyroid monitoring in babies and young children who receive injections of iod ine-
containing contrast media for medical imaging. Available at: https://www.fda.gov/drugs/drug-safety-and-availability/fda-recommends-thyroid-
monitoring-babies-and-young-children-who-receive-injections-iodine-
containing#:~:text=Therefore%2C%20FDA%20recommends%20that%20newborns,many%20of%20the%20body's%20functions. Accessed
May, 2024.
25.
Dillman JR, Ellis JH, Cohan RH, Strouse PJ, Jan SC. Frequency and severity of acute allergic -like reactions to gadolinium-containing i.v.
contrast media in children and adults. AJR Am J Roentgenol 2007;189:1533-8.
26.
Davenport MS, Dillman JR, Cohan RH, et al. Effect of abrupt substitution of gadobenate dimeglumine for gadopentetate dimeglum ine on rate of
allergic-like reactions. Radiology 2013;266:773-82.
27.
Forbes-Amrhein MM, Dillman JR, Trout AT, et al. Frequency and Severity of Acute Allergic-Like Reactions to Intravenously Administered
Gadolinium-Based Contrast Media in Children. Invest Radiol 2018;53:313-18.
28.
Nardone B, Saddleton E, Laumann AE, et al. Pediatric nephrogenic systemic fibrosis is rarely reported: a RADAR report. Pediat r Radiol
2014;44:173-80.
29.
Penfield JG. Nephrogenic systemic fibrosis and the use of gadolinium-based contrast agents. Pediatr Nephrol 2008;23:2121-9.
30.
Smeets NJL, IntHout J, van der Burgh MJP, Schwartz GJ, Schreuder MF, de Wildt SN. Maturation of GFR in Term -Born Neonates: An
Individual Participant Data Meta-Analysis. J Am Soc Nephrol 2022;33:1277-92.
31.
Rossi Espagnet MC, Bernardi B, Pasquini L, Figà-Talamanca L, Tomà P, Napolitano A. Signal intensity at unenhanced T1-weighted magnetic
resonance in the globus pallidus and dentate nucleus after serial administrations of a macrocyclic gadolinium -based contrast agent in children.
Pediatr Radiol 2017;47:1345-52.
32.
Topcuoglu ED, Topcuoglu OM, Semiz Oysu A, Bukte Y. Does Gadoterate Meglumine Cause Gadolinium Retention in the Brain of Child ren? A
Case-Control Study. J Magn Reson Imaging 2020;51:1471-77.
33.
Ichikawa S, Omiya Y, Onishi H, Motosugi U. Linear gadolinium-based contrast agent (gadodiamide and gadopentetate dimeglumine)-induced
high signal intensity on unenhanced T(1) -weighted images in pediatric patients. J Magn Reson Imaging 2019;49:1046-52.
34.
Pozeg P, Forget J, Meuli RA, Maeder P. Age, But Not Repeated Exposure to Gadoterate Meglumine, Is Associated With T1- and T2-Weighted
Signal Intensity Changes in the Deep Brain Nuclei of Pediatric Patients. Invest Radiol 2019;54:537-48.
35.
Radbruch A, Haase R, Kickingereder P, et al. Pediatric Brain: No Increased Signal Intensity in the Dentate Nucleus on Unenhan ced T1-weighted
MR Images after Consecutive Exposure to a Macrocyclic Gadolinium-based Contrast Agent. Radiology 2017;283:828-36.
36.
Ryu YJ, Choi YH, Cheon JE, et al. Pediatric Brain: Gadolinium Deposition in Dentate Nucleus and Globus Pallidus on Unenhanced T1-
Weighted Images Is Dependent on the Type of Contrast Agent. Invest Radiol 2018;53:246-55.
37.
Schneider GK, Stroeder J, Roditi G, et al. T1 Signal Measurements in Pediatric Brain: Findings after Multiple Exposures to Ga dobenate
Dimeglumine for Imaging of Nonneurologic Disease. AJNR Am J Neuroradiol 2017;38:1799-806.
38.
Tamrazi B, Liu CJ, Cen SY, Nelson MB, Dhall G, Nelson MD. Brain Irradiation and Gadobutrol Administration in Pediatric Patien ts with Brain
Tumors: Effect on MRI Brain Signal Intensity. Radiology 2018;289:188-94.
39.
Young JR, Orosz I, Franke MA, et al. Gadolinium deposition in the paediatric brain: T1-weighted hyperintensity within the dentate nucleus
following repeated gadolinium-based contrast agent administration. Clin Radiol 2018;73:290-95.
40.
Colafati GS, Rossi E, Carducci C, et al. Half-dose versus full-dose macrocyclic gadolinium at 3-T magnetic resonance imaging in paediatric bone
and soft-tissue disease. Pediatr Radiol 2018;48:1724-35.
41.
Callahan MJ, Talmadge JM, MacDougall RD, Kleinman PL, Taylor GA, Buonomo C. Selecting appropriate gastroenteric contrast media for
diagnostic fluoroscopic imaging in infants and children: a practical approach. Pediatr Radiol 2017;47:372-81.
42.
Yamamura M, Nishi M, Furubayashi H, Hioki K, Yamamoto M. Barium peritonitis. Report of a case and review of the literature. Dis Colon
Rectum 1985;28:347-52.
43.
Chiu CY, Wong KS, Tsai MH. Massive aspiration of barium sulfate during an upper gastrointestinal examination in a child with dysphagia. Int J
Pediatr Otorhinolaryngol 2005;69:541-4.
44.
McAlister WH, Siegel MJ. Fatal aspirations in infancy during gastrointestinal series. Pediatr Radiol 1984;14:81-3.
45.
Skucas J. Anaphylactoid reactions with gastrointestinal contrast media. AJR Am J Roentgenol 1997;168:962-4.
46.
Harris PD, Neuhauser EB, Gerth R. The Osmotic Effect of Water Soluble Contrast Media on Circulating Plasma Volume. Am J Roent genol
Radium Ther Nucl Med 1964;91:694-8.
47.
Johansen JG, Kolmannskog S. Osmotic effect and solubility of amipaque (metrizamide) in the gastrointestinal tract. Invest Radiol 1978;13:93-7.
48.
Cohen MD. Choosing contrast media for the evaluation of the gastrointestinal tract of neonates and infants. Radiology 1987;162:447-56.
49.
Poole CA, Rowe MI. Clinical evidence of intestinal absorption of Gastrografin. Radiology 1976;118:151-3.
50.
Rowe MI, Seagram G, Weinberger M. Gastrografin-induced hypertonicity. The pathogenesis of a neonatal hazard. Am J Surg 1973;125:185-8.
51.
Long AM, Jones IH, Knight M, McNally J. Early management of meconium ileus in infants with cystic fibrosis: A prospective population cohort
study. J Pediatr Surg 2021;56:1287-92.
52.
Rubalcava NS, Bence CM, Jensen AR, et al. Contrast Challenge Algorithms for Adhesive Small Bowel Obstructions Are Safe in Children: A
Multi-Institutional Study. Ann Surg 2023;277:e925-e32.
CONTRAST MEDIA IN CHILDREN 56
53.
Koshinaga T, Inoue M, Ohashi K, Sugito K, Ikeda T, Tomita R. Therapeutic strategies of meconium obstruction of the small bowe l in very-low-
birthweight neonates. Pediatr Int 2011;53:338-44.
54.
Shin J, Jeon GW. Successful Ultrasound-Guided Gastrografin Enema for Very Low Birth Weight Infants with Meconium-Related Ileus.
Neonatal Med 2018;25:37-43.
55.
Mitani Y, Kubota A, Goda T, et al. Optimum therapeutic strategy for meconium-related ileus in very-low-birth-weight infants. J Pediatr Surg
2021;56:1117-20.
56.
Frech RS, Davie JM, Adatepe M, Feldhaus R, McAlister WH. Comparison of barium sulfate and oral 40 per cent diatrizoate inject ed into the
trachea of dogs. Radiology 1970;95:299-303.
57.
Friedman BI, Hartenberg MA, Mulroy JJ, Tong TK, Mickell JJ. Gastrografin aspiration in a 3 3/4-year-old girl. Pediatr Radiol 1986;16:506-7.
58.
Reich SB. Production of pulmonary edema by aspiration of water-soluble nonabsorbable contrast media. Radiology 1969;92:367-70.
59.
Dillman JR, Towbin AJ, Imbus R, Young J, Gates E, Trout AT. Comparison of Two Neutral Oral Contrast Agents in Pediatric Patients: A
Prospective Randomized Study. Radiology 2018;288:245-51.
60.
Young BM, Fletcher JG, Booya F, et al. Head-to-head comparison of oral contrast agents for cross-sectional enterography: small bowel
distention, timing, and side effects. J Comput Assist Tomogr 2008;32:32-8.
CONTRAST MEDIA IN CHILDREN 57
Ferumoxytol as MRI Contrast Medium
Ferumoxytol (Feraheme; AMAG Pharmaceuticals, Waltham, MA and ferumoxytol; Sandoz Inc., Princeton, NJ) is approved by
the Food and Drug Administration (FDA) for intravenous (IV) treatment of iron deficiency anemia in adults [1 -6]. However,
off-label use of this iron oxide nanoparticle compound as a magnetic resonance imaging (MRI) contrast medium is growing
[7,8]. Benefits of ferumoxytol as an intravenous MRI imaging medium include a prolonged blood pool phase and delayed
intracellular uptake, allowing use as a pure intravascular contrast medium, and high T1 and T2 relaxivities compared to
gadolinium-based contrast media [9]. Ferumoxytol has no known acute nephrotoxicity and may be considered in patients with
severely impaired renal function [5,6].
Uses
Vascular imaging—The long circulating time of ferumoxytol, with a plasma half-life of 14–21 h [10], is exploited for
cardiovascular [11,12] and peripheral vascular MRI examinations [13]. Ferumoxytol has been used in MRA of coronary artery
stenosis [12], abdominal aortic aneurysms [12], renal artery stenosis [14], renal transplant [12], deep vein thrombosis [15],
pulmonary thromboembolism [12], central venous occlusion [16], pediatric cardiovascular imaging, and peripheral arterial
disease [8]. Ferumoxytol has also been used for steady-state [17-19] and dynamic [20] cerebral blood volume mapping of the
brain.
Delayed phase imaging — Slow leakage of ferumoxytol from blood vessels results in intracellular MRI signal changes peaking
around 24 h after administration. Signal decrease on T2-weighted and T2*-weighted images may represent accumulation of iron
stores, macrophage or phagocytic uptake, and/or sequestration in tissue interstitium. This characteristic has useful applications
in central nervous system (CNS) lesions [21,22], abdominal organs [23], atherosclerotic plaques [24], and evaluation for
endoleaks after aortic aneurysm stent-graft repair [12]. Macrophage uptake of ferumoxytol allows the assessment of
macrophage activity and identify localized cellular inflammation [25]. Thus, the immune response to tumors, such as gliomas
and lymphomas, may enable the imaging of tumor extent. Macrophage influx related to stem cell death or immune-mismatched
stem cell transplants can be imaged in stem cell transplant rejection [26]. Similarly, ferumoxytol uptake in macrophages has
been associated with instability and impending rupture of vascular lesions, including intracranial aneurysms, arteriovenous
malformations, and carotid plaques [22]. Conversely, lymph nodes replaced by metastatic tumor may show reduced or absent
uptake [27].
Ferumoxytol administration for MRI Imaging
Imaging with ferumoxytol can be performed at both 1.5 and 3 Tesla [28].
Diagnostic imaging use of ferumoxytol differs from therapeutic use in two important ways: 1) lower total iron dose with
dilution, and 2) lower average injection rate. Typical ferumoxytol doses for imaging range from 1 to 7.5 mg/kg, with most doses
between 2 and 4 mg/kg in both adults and children [29,30]; whereas the standard therapeutic dose for anemia is 1020 mg (14.6
mg/kg for a 70-kg adult over 3-8 day period).
Injection rates reflect specific imaging indications and range from a slow infusion (for lymph node and steady-state imaging) to
bolus injections of 3–15 sec for angiographic applications [30]. All reporting groups dilute the administered dose with saline
(total volume of ~24–60 mL for adults). High injection rates and concentrations may be limited by artifacts from T2* effects.
In the therapeutic ferumoxytol boxed warning, the FDA recommends a slow intravenous infusion over at least 15 minutes of a
diluted medium [31]. For imaging, the ferumoxytol is diluted to 1 part ferumoxytol in ~2-4 parts normal saline, resulting in a
concentration of no higher than 10 mg/mL [30]. However, the infusion time varies depending on the imaging indication.
As with other contrast media, injection should be performed in a setting with personnel and therapies immediately available for
the treatment of anaphylaxis and other hypersensitivity reactions [29]. For therapeutic ferumoxytol administration, FDA
recommends close monitoring of patients for signs and symptoms of hypersensitivity reaction, including monitoring blood
pressure and heart rate during the infusion and for at least 30 minutes after completion of the infusion [29,32].
Pharmacodynamics and pharmacokinetics
Ferumoxytol can be administered as a bolus, allowing dynamic imaging of the arterial vasculature and first-pass perfusion of
the liver and kidneys [33]. There is slow migration of the ferumoxytol across the capillary endothelium (12-14 hours), so
imaging in this time frame allows for venography [34]. The iron-oxide nanoparticles are then sequestered and cleared by the
CONTRAST MEDIA IN CHILDREN 58
mononuclear phagocytic system (MPS) [35], which includes liver, spleen, and bone marrow, and to a lesser extend lymph
nodes. As the cells of MPS accumulated iron, they progressively lose T2- and T2*-weighted signal intensity. Iron deposition
in the mononuclear phagocytic system (liver, spleen, and bone marrow) can cause signal change on MRI for up to 11 months
[22,35,36], which may affect tissue and lesion characterization on subsequent MRI examinations.
The body has no active excretory pathways to remove excess iron from administration. For MRI, the amount of contrast
administered (<4 mg/kg) is much lower than doses resulting in acute systemic toxicity (>60 mg/kg) or chronic iron overload
[36]. However, evaluation for preexisting iron overload could be considered in high-risk groups, either through liver T2*
measurements or serum ferritin levels.
As mentioned above, ferumoxytol is FDA approved for therapeutic use in adult patients with iron deficiency anemia in the
setting of chronic kidney disease [6]. It is important for the radiologist to be aware of the common interactions of ferumoxytol
and other intravenous iron formulations on subsequent MR imaging, particularly those studies that are performed within 72
hours of administration.
Ferumoxytol and other ultrasmall superparamagnetic iron oxide compounds result in T1, T2, and T2* shortening effects on
MRI. These phenomena are used clinically for vascular imaging and other specific clinical indications [37], but can also result
in unwanted artifacts, and may impact image interpretation [38]. Maximum intravascular signal alterations occur within 2 days
of administration. The effects of T1 shortening and prolonged intravascular half-life of ferumoxytol can also result in
interactions with conventional gadolinium-based contrast media for MRI [39], occasionally rendering typical enhancement
patterns undetectable if ferumoxytol was administered within 72 hours of the MR examination. The package insert for
ferumoxytol warns of potential prolonged MR effects [40], and can increase the R2* signal in hematopoietic bone marrow for
up to a period of 3 months and liver for up to 6 months [41], mimicking hemosiderosis.
Other parenteral iron formulations are available, but are not used as contrast media. These preparations can also affect MR
signal, and recommended times between infusion and MR imaging have been published [41]. There may be usefulness in
reimaging with MRI soon after parenteral iron administration, however, the interpreting radiologist should be aware that MR
signal may be impacted in this time frame. In brain pathologies, enhancement can persist for several days following
ferumoxytol administration [35]. Ferumoxytol has no effect on radiography, computed tomography, positron emission
tomography, single photon emission computed tomography, ultrasound, or nuclear medicine imaging [42].
Safety profile
Serious and rarely fatal adverse reactions to therapeutic doses of ferumoxytol, including anaphylaxis associated with
cardiac/cardiorespiratory arrest, have been reported. From 2009 to 2015, approximately 1.2 million therapeutic doses of
ferumoxytol were administered for treatment of iron deficiency anemia. In March 2015, the US Food & Drug Administration
(FDA) placed a boxed warning on ferumoxytol for serious, potentially fatal allergic reactions after the FDA Adverse Event
Reporting System showed 79 anaphylactic reactions, with 18 fatalities despite immediate intervention [31]. Twenty-four
percent of these patients had multiple drug allergies, and nearly half of these anaphylactic reactions occurred within 5 minutes
of administration.
The largest experience for diagnostic use of ferumoxytol is a retrospective, observational study using data from the FeraSafeTM
Multicenter MRI Registry, consisting of 11 institutional partners in the U.S. and U.K. [43]. Between 2003 and 2018, a total of
3,215 unique patients (13% children, 87% adults) had 4,240 ferumoxytol injections for MRI. Ferumoxytol dose ranged from 1-
11mg/kg (≤510mg Fe; rate ≤45mg Fe/s). There were no systematic changes reported in heart rate, blood pressure or oxygen
saturation following ferumoxytol administration. No severe, life-threatening, or fatal adverse events occurred. Eighty-three
adverse events (1.9%) were related or possibly related to ferumoxytol infusions (1.8 % mild; 0.2% moderate). Thirty-one were
classified as allergic-like using ACR criteria but were consistent with known minor infusion reactions observed with parenteral
iron. Other studies have shown similar results of an overall adverse event rate of 2%, serious hypersensitivity reactions in
0.01% of patients, and no reports of death [44].
Similar to other contrast media, hypersensitivity/allergic-like reactions may occur after the first dose or subsequent doses, even
in patients who have previously tolerated the drug [1]. Patients with a history of multiple medication allergies have an increased
risk of hypersensitivity reaction to parenteral iron preparations, therefore, potential risks and benefits of ferumoxytol
administration should be considered carefully in such patients. The current FDA recommendation is that ferumoxytol should
not be administered to patients with a history of allergic reactions to IV iron preparations [29,45].

CONTRAST MEDIA IN CHILDREN 59
A study in pigs showed no significant differences in the brain MRI of pigs exposed to ferumoxytol doses of 5-10 mg/kg
compared to unexposed pigs [15]. A study of 17 pediatric patients who received between one and four doses of ferumoxytol for
evaluation of arteriovenous malformations were compared to control patients, and showed no differences in susceptibility and
R* values of the deep gray structures of the brain between the two groups [46].
Pregnant and breastfeeding patients
There is insufficient available data about ferumoxytol use in pregnant women to make conclusions regarding any risk of adverse
developmental outcomes. There are risks to the mother and fetus associated with maternal severe hypersensitivity reactions
[47,48]. Severe adverse reactions, including severe hypotension and shock, may occur in pregnant women with parental iron
products which may cause fetal bradycardia, especially during the second and third trimester [1,49].
In animal studies, administration of ferumoxytol to pregnant rabbits at doses up to 2 times the estimated therapeutic human
daily dose during organogenesis did not result in maternal or fetal effects [1]. In rabbits and rats, administration of ferumoxytol
during organogenesis at approximately 6 times the estimated human daily dose caused adverse developmental outcomes
including fetal malformations and decreased fetal weights [1]. One study that looked at ferumoxytol administration in rhesus
macaques for MRI placental imaging showed placental ferumoxytol retention similar to levels of maternal liver ferumoxytol
retention, which returned to baseline by day 4 post injection [50]. No increase in ferumoxytol was measured in the fetal tissues
or maternal-fetal interface (decidua, placenta, fetal membranes) on MRI or post-delivery histopathologic evaluation, suggesting
ferumoxytol may not enter the fetal circulation. Placental function, as measured by peripheral blood steroid hormone levels,
was unaffected.
No data is available on the presence of ferumoxytol in human milk, the effects on breastfed children, or the effects on milk
production.
REFERENCES
1.
FDA. Full prescribing information: fereheme. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/022180s009lbl.pdf
Accessed May, 2024.
2.
Sandoz. Sandoz launches first generic high dose intravenous iron, Ferumoxytol Injection, to treat US patients with iron deficiency anemia.
Available at: https://www.sandoz.com/news/media-releases/sandoz-launches-first-generic-high-dose-intravenous-iron-ferumoxytol-injection.
Accessed May, 2024.
3.
FDA. FDA-Approved Drug: Sandoz inc. Ferumoxytol. . Available at:
https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2021/206604Orig1s000ltr.pdf. Accessed May, 2024.
4.
Spinowitz BS, Kausz AT, Baptista J, et al. Ferumoxytol for treating iron deficiency anemia in CKD. J Am Soc Nephrol 2008;19:1599-605.
5.
Provenzano R, Schiller B, Rao M, Coyne D, Brenner L, Pereira BJ. Ferumoxytol as an intravenous iron replacement therapy in hemodialysis
patients. Clin J Am Soc Nephrol 2009;4:386-93.
6.
Singh A, Patel T, Hertel J, Bernardo M, Kausz A, Brenner L. Safety of ferumoxytol in patients with anemia and CKD. Am J Kidney Dis
2008;52:907-15.
7.
Mukai K, Burris NS, Mahadevan VS, Foster ED, Ordovas KG, Hope MD. 4D flow image quality with blood pool contrast: a comparison of
gadofosveset trisodium and ferumoxytol. Int J Cardiovasc Imaging 2018;34:273-79.
8.
Walker JP, Nosova E, Sigovan M, et al. Ferumoxytol-enhanced magnetic resonance angiography is a feasible method for the clinical evaluation
of lower extremity arterial disease. Ann Vasc Surg 2015;29:63-8.
9.
Bashir MR, Bhatti L, Marin D, Nelson RC. Emerging applications for ferumoxytol as a contrast agent in MRI. J Magn Reson Imaging
2015;41:884-98.
10.
Pai AB, Nielsen JC, Kausz A, Miller P, Owen JS. Plasma pharmacokinetics of two consecutive doses of ferumoxytol in healthy su bjects. Clin
Pharmacol Ther 2010;88:237-42.
11.
Prince MR, Zhang HL, Chabra SG, Jacobs P, Wang Y. A pilot investigation of new superparamagnetic iron oxide (ferumoxytol) as a contrast
agent for cardiovascular MRI. J Xray Sci Technol 2003;11:231-40.
12.
Hope MD, Hope TA, Zhu C, et al. Vascular Imaging With Ferumoxytol as a Contrast Agent. AJR Am J Roentgenol 2015;205:W366-73.
13.
Anzai Y, Prince MR, Chenevert TL, et al. MR angiography with an ultrasmall superparamagnetic iron oxide blood pool agent. J Magn Reson
Imaging 1997;7:209-14.
14.
Ersoy H, Jacobs P, Kent CK, Prince MR. Blood pool MR angiography of aortic stent-graft endoleak. AJR Am J Roentgenol 2004;182:1181-6.
15.
Bashir MR, Mody R, Neville A, et al. Retrospective assessment of the utility of an iron -based agent for contrast-enhanced magnetic resonance
venography in patients with endstage renal diseases. J Magn Reson Imaging 2014;40:113-8.
16.
Shahrouki P, Moriarty JM, Khan SN, et al. High resolution, 3-dimensional Ferumoxytol-enhanced cardiovascular magnetic resonance
venography in central venous occlusion. J Cardiovasc Magn Reson 2019;21:17.
17.
Christen T, Ni W, Qiu D, et al. High-resolution cerebral blood volume imaging in humans using the blood pool contrast agent ferumoxytol.
Magn Reson Med 2013;70:705-10.
18.
Rivera-Rivera LA, Schubert T, Knobloch G, et al. Comparison of ferumoxytol-based cerebral blood volume estimates using quantitative R(1)
and R2* relaxometry. Magn Reson Med 2018;79:3072-81.
19.
Varallyay CG, Nesbit E, Fu R, et al. High-resolution steady-state cerebral blood volume maps in patients with central nervous system neoplasms
using ferumoxytol, a superparamagnetic iron oxide nanoparticle. J Cereb Blood Flow Metab 2013;33:780-6.
20.
Varallyay CG, Nesbit E, Horvath A, et al. Cerebral blood volume mapping with ferumoxytol in dynamic susceptibility contrast perfusion MRI:

CONTRAST MEDIA IN CHILDREN 60
Comparison to standard of care. J Magn Reson Imaging 2018;48:441-48.
21.
Hamilton BE, Barajas R, Nesbit GM, et al. Ferumoxytol-Enhanced MRI Is Not Inferior to Gadolinium-Enhanced MRI in Detecting Intracranial
Metastatic Disease and Metastasis Size. AJR Am J Roentgenol 2020;215:1436-42.
22.
Hasan DM, Amans M, Tihan T, et al. Ferumoxytol-enhanced MRI to Image Inflammation within Human Brain Arteriovenous Malformations: A
Pilot Investigation. Transl Stroke Res 2012;3:166-73.
23.
Shahrouki P, Felker ER, Raman SS, Jeong WK, Lu DS, Finn JP. Steady-state ferumoxytol-enhanced MRI: early observations in benign
abdominal organ masses and clinical implications. Abdom Radiol (NY) 2022;47:460-70.
24.
Smits LP, Tiessens F, Zheng KH, Stroes ES, Nederveen AJ, Coolen BF. Evaluation of ultrasmall superparamagnetic iron -oxide (USPIO)
enhanced MRI with ferumoxytol to quantify arterial wall inflammation. Atherosclerosis 2017;263:211-18.
25.
Stirrat CG, Alam SR, MacGillivray TJ, et al. Ferumoxytol-enhanced magnetic resonance imaging methodology and normal values at 1.5 and 3T.
J Cardiovasc Magn Reson 2016;18:46.
26.
Nejadnik H, Tseng J, Daldrup-Link H. Magnetic resonance imaging of stem cell-macrophage interactions with ferumoxytol and ferumoxytol-
derived nanoparticles. Wiley Interdiscip Rev Nanomed Nanobiotechnol 2019;11:e1552.
27.
Muehe AM, Siedek F, Theruvath AJ, et al. Differentiation of benign and malignant lymph nodes in pediatric patients on ferumoxytol-enhanced
PET/MRI. Theranostics 2020;10:3612-21.
28.
Wells SA, Schubert T, Motosugi U, et al. Pharmacokinetics of Ferumoxytol in the Abdomen and Pelvis: A Dosing Study with 1.5- and 3.0-T
MRI Relaxometry. Radiology 2020;294:108-16.
29.
Nguyen KL, Yoshida T, Han F, et al. MRI with ferumoxytol: A single center experience of safety across the age spectrum. J Magn Reson
Imaging 2017;45:804-12.
30.
Vasanawala SS, Nguyen KL, Hope MD, et al. Safety and technique of ferumoxytol administration for MRI. Magn Reson Med 2016;75:2107-11.
31.
FDA. FDA Drug Safety Communication: FDA strengthens warnings and changes prescribing instructions to decrease the risk of ser ious allergic
reactions with anemia drug Feraheme (ferumoxytol). Available at: https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-
communication-fda-strengthens-warnings-and-changes-prescribing-instructions-decrease. Accessed May, 2024.
32.
Iv M, Ng NN, Nair S, et al. Brain Iron Assessment after Ferumoxytol-enhanced MRI in Children and Young Adults with Arteriovenous
Malformations: A Case-Control Study. Radiology 2020;297:438-46.
33.
Schubert T, Motosugi U, Kinner S, et al. Crossover comparison of ferumoxytol and gadobenate dimeglumine for abdominal MR -angiography at
3.0 tesla: Effects of contrast bolus length and flip angle. J Magn Reson Imaging 2017;45:1617-26.
34.
Han F, Rapacchi S, Khan S, et al. Four-dimensional, multiphase, steady-state imaging with contrast enhancement (MUSIC) in the heart: a
feasibility study in children. Magn Reson Med 2015;74:1042-9.
35.
Storey P, Lim RP, Chandarana H, et al. MRI assessment of hepatic iron clearance rates after USPIO administration in healthy adults. Invest
Radiol 2012;47:717-24.
36.
Weinstein JS, Varallyay CG, Dosa E, et al. Superparamagnetic iron oxide nanoparticles: diagnostic magnetic resonance imaging and potential
therapeutic applications in neurooncology and central nervous system inflammatory pathologies, a review. J Cereb Blood Flow Metab
2010;30:15-35.
37.
Toth GB, Varallyay CG, Horvath A, et al. Current and potential imaging applications of ferumoxytol for magnetic resonance imaging. Kidney
International 2017;92:47-66.
38.
Schieda N. Parenteral ferumoxytol interaction with magnetic resonance imaging: a case report, review of the literature and advisory warning.
Insights Imaging 2013;4:509-12.
39.
McCullough BJ, Kolokythas O, Maki JH, Green DE. Ferumoxytol in clinical practice: implications for MRI. J Magn Reson Imaging
2013;37:1476-9.
40.
Pharmaceuticals A. Feraheme (ferumoxytol) Prescribing Information. Available at:
https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/022180lbl.pdf. Accessed May, 2024.
41.
Rostoker G, Cohen Y. Magnetic Resonance Imaging Repercussions of Intravenous Iron Products Used for Iron -Deficiency Anemia and Dialysis-
Associated Anemia. Journal of Computer Assisted Tomography 2014;38:843-44.
42.
Pharmaceuticals A. Feraheme® (ferumoxytol injection) Important Safety Information - Radiologist Letter. Available at:
https://www.feraheme.com/wp-content/uploads/2020/07/Radiologist-Letter.pdf. Accessed May, 2024.
43.
Nguyen KL, Yoshida T, Kathuria-Prakash N, et al. Multicenter Safety and Practice for Off-Label Diagnostic Use of Ferumoxytol in MRI.
Radiology 2019;293:554-64.
44.
Ahmad F, Treanor L, McGrath TA, Walker D, McInnes MDF, Schieda N. Safety of Off-Label Use of Ferumoxtyol as a Contrast Agent for MRI:
A Systematic Review and Meta-Analysis of Adverse Events. J Magn Reson Imaging 2021;53:840-58.
45.
Varallyay CG, Toth GB, Fu R, et al. What Does the Boxed Warning Tell Us? Safe Practice of Using Ferumoxytol as an MRI Contrast Agent.
AJNR Am J Neuroradiol 2017;38:1297-302.
46.
Theruvath AJ, Aghighi M, Iv M, et al. Brain iron deposition after Ferumoxytol-enhanced MRI: A study of Porcine Brains. Nanotheranostics
2020;4:195-200.
47.
McCall SJ, Bunch KJ, Brocklehurst P, et al. The incidence, characteristics, management and outcomes of anaphylaxis in pregnan cy: a
population-based descriptive study. Bjog 2018;125:965-71.
48.
McCall SJ, Bonnet MP, Äyräs O, et al. Anaphylaxis in pregnancy: a population-based multinational European study. Anaesthesia 2020;75:1469-
75.
49.
Woodward T, Kay T, Rucklidge M. Fetal bradycardia following maternal administration of low -molecular-weight intravenous iron. Int J Obstet
Anesth 2015;24:196-7.
50.
Nguyen SM, Wiepz GJ, Schotzko M, et al. Impact of ferumoxytol magnetic resonance imaging on the rhesus macaque maternal-fetal interface†.
Biol Reprod 2020;102:434-44.
GASTROINTESTINAL (GI) CONTRAST MEDIA IN ADULTS: INDICATIONS AND GUIDELINES 61
GASTROINTESTINAL (GI) CONTRAST MEDIA IN ADULTS:
INDICATIONS AND GUIDELINES
Introduction
This chapter discusses indications, contraindications, and adverse reactions resulting from the administration of contrast agents
used to assess the gastrointestinal system. Barium, iodinated, and negative GI contrast agents will be primarily discussed.
Ancillary drugs utilized in gastrointestinal tract imaging will also be reviewed along with contraindications and risks. Allerg ic-
like reactions and premedication strategies are covered at the end of the chapter.
Barium-based and iodinated contrast agents are commonly used to opacify the bowel lumen during fluoroscopic, computed
tomography (CT), or magnetic resonance imaging (MRI) of the gastrointestinal system. Oral or rectal administration may be
during the study (e.g., during fluoroscopic examinations), or in advance (e.g., prior to CT or MRI). Less commonly, contrast
media can enter the bowel following intravenous injection and hepatobiliary clearance (e.g., with vicarious excretion of iodinated
contrast, or following injection of gadobenate dimeglumine or gadoxetate disodium), or after direct injection of contrast media
into the biliary and pancreatic ducts (e.g., during ERCP).
Barium Sulfate Enteric Contrast Media
Barium sulfate is a micropulverized white powder that is supplied in various forms, including in bulk for mixing with distilled or
tap water, in prepackaged aliquots ready for suspension, or in prepared doses. Barium sulfate has a long history of use in diagnostic
imaging, having been first described as a gastrointestinal contrast medium in 1910. Barium was the default enteric contrast
medium for the evaluation of the GI tract for almost a century, and it retains value with both fluoroscopy and cross-sectional
imaging.
Barium Sulfate in Fluoroscopy
Barium sulfate preparations remain the contrast media of choice for fluoroscopic imaging due to the delineation of mucosal detail
and resistance to dilution. This is particularly helpful when performing double contrast studies and studies of swallowing
mechanics using differing consistencies.
During a single contrast fluoroscopic examination, the optimal mixture for stability in suspension and bowel wall coating is 60%
weight/volume (w/v) [1]. The volume of barium required varies by procedure and individual patient anatomy. For double contrast
GI studies, high density barium (up to 250% w/v) is used in conjunction with air or effervescent material with a suspension of 85-
100% weight/volume recommended for optimal double contrast imaging in the colon [2].
The composition of barium mixtures is altered in different areas of the gastrointestinal tract by local conditions including the
presence of fluid and luminal acidity, which affects flocculation (clumping out of suspension) and coating. Also, local differences
in tap water composition obtained from municipal sources alter the qualities of barium, so that trial and error may be needed to
obtain the optimal constitution [3].
Water-soluble contrast media (discussed below) are generally preferred as oral contrast agents when a bowel perforation is
suspected or known to exist. If an initial study with an iodinated contrast medium fails to demonstrate a suspected perforation,
barium sulfate can then be administered. Such follow-up studies may be important as some small leaks may be undetectable with
water-soluble media but subsequently seen when using barium-based media [4,5].
Barium Sulfate in Cross Sectional Imaging
Extremely dilute barium sulfate solutions are commonly used for opacification of the GI tract with CT, and less commonly with
PET/CT. Its use varies among radiology departments, with some opacifying the GI tract routinely and others seldom or for specific
indications only. The principal disadvantage is the time taken to opacify the GI tract which becomes relevant in emergency ca re
[6,7]. Many feel that its routine use cannot be justified as it is seldom necessary to repeat a study with oral contrast following a
study without it [7,8]. Targeted utilization (for example, for postoperative evaluation or in, patients who cannot receive
intravenous (IV) contrast) is often suggested. Barium sulfate is avoided in patients with suspected perforation and known barium
allergy in favor of water-soluble contrast (see below). There is no difference in opacification of the bowel on CT with barium
sulfate or water-soluble alternatives [9].
GASTROINTESTINAL (GI) CONTRAST MEDIA IN ADULTS: INDICATIONS AND GUIDELINES 62
For evaluation of the small bowel with CT or MR enterography, routine oral preparations of dilute barium should not be used as
they may obscure mural or mucosal abnormalities due to the density. Instead, negative contrast agents are often used (discussed
below).
Non-Allergic Complications of Barium Sulfate
The most serious complication from the use of barium in the GI tract is leakage into the mediastinum or peritoneal cavity [10].
The potential complications of a barium leak depend on the site from which the spill occurs. Esophageal leakage may cause
mediastinitis. Gastric, duodenal, and small intestinal leakage may result in peritonitis. Escape of barium from the colon, where
the bacterial count is highest, carries the highest mortality (likely primarily related to leakage of stool). Water-soluble contrast
media (discussed below) are generally preferred as oral contrast agents when a bowel perforation is suspected or known to exist.
If an initial study with an iodinated contrast medium fails to demonstrate a suspected perforation, barium sulfate can then be
administered. Such follow-up studies may be important as some small leaks may be undetectable with water-soluble media but
subsequently seen when using barium-based media [4,5].
Although barium sulfate is inert, it can occasionally produce symptoms if aspirated, particularly in patients who have underlying
lung disease. While barium is usually mobilized proximally by ciliary action of normal bronchial epithelium, damaged epithelium
from bronchial disease delays the normal elimination of barium [1]. If not completely expectorated, retained barium in the lungs
can remain indefinitely and may cause inflammation [11]. High volume aspiration can lead to acute respiratory distress or
pneumonia [12].
Other reactions to GI tract barium
Adverse reactions to oral and rectal barium contrast media are almost always mild, with the most common symptoms including
nausea, vomiting, and abdominal cramping or discomfort during and/or after the examination. These symptoms are likely not
hypersensitivity reactions but are part of a physiologic response resulting from distention of a viscus. Vasovagal reactions can
also be encountered, when the colon is distended during a double contrast barium enema.
Direct barium toxicity
Direct toxicity of orally or rectally administered barium has been reported on very rare occasions [13,14]. Any barium that
dissociates from the stable barium sulfate compound may form other chemical compounds that become soluble and are absorbed
into the blood stream resulting in toxicity – for example, barium chloride, barium sulfide, or barium carbonate [15]. This is more
likely to occur if industrial grade barium contaminates pharmaceutical grade barium distributed for diagnostic use [16]. Case
reports of toxicity with pharmaceutical grade barium also exist [14,17].
Acute symptoms of barium toxicity are usually rapid in onset and include nausea, vomiting, and watery diarrhea. Absorption of
barium can result in changes in electrolyte balance, causing rapid and severe hypokalemia [18,19]. Untreated, this can lead to a
cascade of severe muscle weakness, respiratory arrest, coma, cardiac arrhythmia, and death [16,20]. Therapy consists of aggressive
potassium infusion with monitoring and correction of electrolyte imbalances [16,21].
Contraindications to administration of barium
There are no absolute contraindications for the use of barium compounds, although as noted above, it is generally recommended
that barium not be administered to individuals who are suspected or known to have bowel perforations or suspected allergy to
barium and/or barium components.
Iodinated (Water-Soluble) Enteric Contrast Media
Two commercial iodinated contrast agents are commonly used for enteric opacification: Gastrografin® (Bracco Diagnostics, Inc.;
Princeton, NJ) and Gastroview® (Covidien; Hazelwood, MO) are solutions comprising 660 mg/ml diatrizoate meglumine and
100 mg/ml diatrizoate sodium. The result is a solution that has 367 mg (37%) of iodine per ml [22,23]. Gastrografin® and
Gastroview® are hypertonic solutions and are considered High Osmolar Contrast Media (HOCM).
Low osmolar iohexol (Omnipaque™ GE Healthcare; Princeton, NJ) has an FDA-approved indication for oral use in select
concentrations. Omnipaque™350 is approved for oral or rectal administration in adults, with lower concentrations approved for
pediatric patients. Diluted and oral solutions of iohexol are approved for use in conjunction with intravenous administration of
iohexol for CT imaging of the abdomen (see prescribing information for further detail) [24,25]. Dilution for CT applications aims
to avoid artifacts that may occur from high attenuation of the contrast media.
GASTROINTESTINAL (GI) CONTRAST MEDIA IN ADULTS: INDICATIONS AND GUIDELINES 63
Diagnostic use of water-soluble enteric contrast media for Conventional Fluoroscopic Examinations
As discussed above, barium sulfate formulations are generally the preferred agents for opacification of the gastrointestinal tract
for conventional fluoroscopic examinations [1,2]. The use of water-soluble contrast media during fluoroscopy is typically reserved
for selected situations, including:
•
Possible bowel perforation, postoperative leak, enteric fistula/sinus tract, or abscess
•
Confirmation of feeding tube positioning
•
Prior to planned endoscopic procedures
•
In small bowel obstruction when surgery may be planned
•
In patients with prior allergic-like reactions to barium
Diagnostic use of water-soluble enteric contrast media for CT
Common indications for the use of enterically administered water soluble contrast in Abdominopelvic CT include [26]:
•
Oncologic imaging
•
Suspected bowel leak, fistula, abscess, or fluid collection
•
Abdominal pain
•
CT colonography
Water-soluble contrast media are the preferred agents when evaluating for hollow viscus/bowel perforation because it is rapidly
absorbed in the peritoneum and interstitial spaces. No deleterious effects from the presence of water-soluble contrast media in the
mediastinum, pleural cavity, or peritoneal cavity have been demonstrated [11]. If an initial exam performed with water soluble
contrast in a patient with suspected bowel perforation fails to demonstrate a leak, one can then consider barium sulfate.
Therapeutic use of water-soluble enteric contrast media
Oral iodinated high-osmolality contrast media (HOCM) have been used successfully for the treatment of postoperative adynamic
(or paralytic) ileus and adhesive small-bowel obstruction [27-30]. Given as an enema, HOCM has proved useful in some adults
with barium impaction [31] as well as in patients with cystic fibrosis who have distal intestinal obstruction syndrome (DIOS)
(obstipation) [32]. This is because HOCMs are hypertonic and draw fluid into the bowel lumen.
Non-allergic Complications of water-soluble contrast agents
Diarrhea, nausea, and vomiting may occur following enteric administration of diatrizoate meglumine/diatrizoate sodium solutions
as well as iohexol solutions. Mucosal irritation has also been reported [22,24,25,33]. Mucosal inflammation, mucosal infection,
or bowel obstruction can increase the amount absorbed by several fold [34-36]. As a result, it is not rare to see opacification of
the urinary tract after enteric administration of water-soluble contrast media [37].
Enteric HOCM in hypertonic concentrations may draw fluid into the lumen of the bowel. This can lead to hypovolemia and/or
dehydration, so should also be avoided in patients with fluid and electrolyte imbalances [10,38]. Preparations made from nonionic
LOCM may be preferable for these patients.
Because of the oral route of administration, aspiration is a risk for enteric contrast administration. Low osmolality and iso-osmolar
contrast agents are preferred due to lower risks of life-threatening pulmonary edema if aspirated as compared to high osmolar
agents [39-41]. Further, water soluble contrast agents should be avoided in patients with tracheoesophageal fistula due to risk of
pulmonary complications [22,33].
Thyroid function tests may be altered for variable periods of time [42], even in normal patients, following administration of
iodinated water-soluble biliary contrast agents such as orally administered iopanoate (Telapaque®; Winthrop Pharmaceuticals,
New York, NY) or intravenously administered water-soluble contrast such as iodipamide (Cholografin®; Bracco Diagnostic, Inc.,
Princeton, NJ). Cases of hyperthyroidism have been reported following oral contrast [22,33].
GASTROINTESTINAL (GI) CONTRAST MEDIA IN ADULTS: INDICATIONS AND GUIDELINES 64
Neutral and Negative Enteric Contrast Media
Neutral oral contrast agents (i.e., attenuation or signal intensity similar to water) are used to distend bowel without interfering
with evaluation of mural enhancement. They are commonly used with MR and CT enterography, and to improve evaluation of
the pancreaticobiliary tree.
Negative oral contrast agents (i.e., attenuation or signal intensity less than water) are used to suppress background luminal contents
to improve visualization of the pancreaticobiliary tree.
Some neutral contrast agents contain nonabsorbable additives (resins, polyethylene glycol, mannitol, sorbitol, or other sugar
alcohols) that help retain intraluminal water and prevent absorption. For example, NeuLumEx (formerly VoLumen, Bracco
Diagnostics, Princeton, NJ) is a thin (0.1% w/v) barium sulfate solution approved that is widely used for CT and MRI enterography
and contains a small amount of sorbitol. Sorbitol is hyperosmolar and retains water, improving bowel distention [43,44]. Breeza
(Beekley Medical, Bristol, Conn) is a well-tolerated alternative neutral contrast agent containing sorbitol, mannitol and xantham
gum. It is marketed as a “flavored beverage” and may be better tolerated than NeuLumEx [43,45].
There have been very few reported serious adverse reactions to neutral or negative oral contrast media. Examples include nausea,
cramping, GI distress, and diarrhea. Barium-based solutions are relatively contraindicated in patients with bowel leak due to the
potential for barium peritonitis.
Neutral Enteric Contrast Media with CT
Neutral oral contrast agents used with CT can include dilute barium-based media, water, milk, and dilute iodinated media.
Negative oral contrast agents are not generally used with CT.
Water is the least expensive, safest, and most accessible neutral contrast medium [46]. Water will distend the lumen of the stomach
and proximal duodenum but not the distal small bowel or colon due to interval absorption.
Commercially available neutral contrast agents containing sorbitol and/or mannitol are commonly used for purposes like CT
Enterography. These include NeuLumEx (Bracco Diagnostics, Princeton, NJ) and Breeza (Beekley Medical, Bristol, Conn).
Milk has been tested as an inexpensive neutral oral contrast material for CT [47,48]. Milk contains fat, which slows intestinal
peristalsis and transit. One comparison of whole milk to VoLumen (now NeuLumEX) in 215 adult patients found no significant
difference with respect to bowel distention or bowel wall visualization but better patient acceptance [47].
Other less common neutral oral contrast agents have been used with CT, including lactulose solution, polyethylene glycol (PEG),
and methylcellulose. In general, these uncommon agents are not favored due to side effects such as diarrhea and dehydration.
Neutral and Negative Enteric Contrast Media with MRI
Neutral and negative oral contrast agents are used during MRI to improve visualization of the pancreaticobiliary tree at MRCP
(i.e., by suppressing background fluid within the stomach and small bowel) or improve visualization of the bowel during MR
enterography (i.e., by distending the bowel lumen and enhancing evaluation of the bowel wall). In general, these agents function
via T1 and T2/T2* effects which improve visualization of mucosal enhancement.
Neutral oral contrast agents used at MRI can include paramagnetic agents containing manganese (e.g., pineapple or blueberry
juice). Use of diamagnetic agents (i.e., barium or kaopectate) or superparamagnetic substances (e.g., oral iron) is rare.
Perfluorochemicals (i.e. molecules where protons are replaced by flourine atoms) have been studied but are not in routine clinical
use [49].
Contrast Agents in the Biliary and Pancreatic Ductal Systems
Orally ingested barium contrast material can reflux into the biliary tree following biliary surgery, sphincteroplasty, or biliary stent
placement. Normally this is of no consequence, as the barium empties back into the bowel promptly with physiological bile flow
[50,51]. However, rare clinical reports of complications like suppurative cholangitis, shock, or disseminated intravascular
coagulation exist, particularly when there is retention of barium beyond 24 hours [52-55]. Although some authors have theorized
that barium could dehydrate and occlude the biliary system or indwelling stents, this has not been well documented [51,56].
GASTROINTESTINAL (GI) CONTRAST MEDIA IN ADULTS: INDICATIONS AND GUIDELINES 65
Water-soluble iodinated contrast media (ICM) is instilled into the biliary ductal system during endoscopic retrograde
cholangiopancreatography (ERCP), percutaneous transhepatic cholangiography, or during intraoperative cholangiography.
Studies have demonstrated that small amounts may be absorbed and result in systemic exposure [57-59]. However,
hypersensitivity reactions to ICM instilled during ERCP are rare [60-63]. Opacification of the biliary tree can also be achieved
via intravenous injection of iodipamide (Cholografin®, Bracco Diagnostics, Milan, Italy) before fluoroscopy or CT, although this
is now rarely (if ever) performed in the United States due to increased utilization of MRCP and the higher frequency of contrast
reactions with this agent [64-67].
Two commonly used gadolinium-based contrast agents (GBCA) – gadobenate dimegumine (MultiHance® Bracco Diagnostics,
Inc., Location) and gadoxetate disodium (Eovist®; Bayer Healthcare Pharmaceuticals, Wayne, NJ) – also have properties that
result in transportation into hepatocytes and subsequent excretion into the biliary tree [68-70]. There are also small case series
describing retrograde injection of GBCA into the pancreaticobiliary tree during ERCP, although there is limited research on
resulting adverse events and the potential for absorption and systemic exposure [62,71].
Ancillary drugs
Glucagon and Anticholinergic Agents
Glucagon is FDA-approved to temporarily inhibit movement of the gastrointestinal tract in adults undergoing radiologic
examinations [72-74]. Most often, glucagon is used to relax the bowel during double contrast studies of the upper GI tract or
colon, or during MR enterography, although it can also be advantageous during intravascular or image-guided percutaneous
procedures [75-79]. The dose in adults can depend on which portion of the GI tract is targeted for relaxation; beneficial effects
are seen in the stomach with doses of 0.5 mg IV (or 2 mg IM), in small bowel with doses of 0.1 to 0.25 mg IV (or 1 mg IM), and
in colon with doses of 1 mg IV (or 2 mg IM given 10 minutes in advance) [76,80]. A standard dose of 0.5-1 mg IV glucagon is
commonly used for MR enterography in order to minimize motion artifacts from bowel peristalsis. Onset occurs within 1 minute
when given IV or within 10 minutes when given IM and lasts at least 30 minutes [81-83]. Side effects of IV glucagon include
nausea and vomiting which can be reduced by slow administration over 1 to 5 minutes [78], as well as vasovagal reactions [73].
Delayed hypoglycemia has been documented in some patients [78], although this is usually not clinically significant. Notably,
some package inserts for glucagon (for example, GlucaGen; Bedford Laboratories; Bedford, OH) recommend oral carbohydrates
after administration of glucagon to rebuild body glycogen stores and avoid hypoglycemia. The package insert for the specific drug
used at each practice should be consulted.
Other anticholinergic agents like hyoscyamine sulfate and hyoscine butylbromide (scopolamine) have been explored as alternative
options to IV glucagon due to cost and side effect profiles. One study found that using sublingual hyoscyamine tablets resulted in
subjectively equivalent quality of upper GI examinations when compared to IV glucagon [84]. Another study found no significant
difference in colonic distension or abdominal discomfort when using sublingual hyoscyamine, although less nausea was reported
by patients [73]. However, other studies have shown lower effectiveness and overall reduced the use of hyoscyamine [73,85-88].
Metoclopramide
Metoclopramide (Reglan®; Pfizer, New York, NY) can be administered to stimulate motility of the upper GI tract and relax the
pyloric sphincter without stimulating gastric, biliary, or pancreatic secretions. This can be helpful to hasten gastric empty ing and
small bowel transit or ease the placement of post-pyloric feeding tubes. Metoclopramide can be given at doses of 10 mg IV
(slowly, over 1-2 minutes), 10 mg IM, or 20 mg PO. The onset of action is 1 to 3 minutes with IV dosing, 10 to 15 minutes with
IM dosing, and 20 to 60 minutes following an oral dose. Pharmacological effects persist for 1 to 2 hours [89,90].
Contraindications to metoclopramide include pheochromocytoma, as it may stimulate release of catecholamines from the tumor.
Epileptics may also be sensitive to its extrapyramidal effects [91]. Other adverse reactions from single doses as given in a radiology
department are exceedingly rare. While adverse reactions from larger or regular dosing exist (e.g., tardive dyskinesia), this is
outside of the scope of this document.
Secretin
A synthetic version of secretin (ChiRhoClin®, Inc., Silver Springs, MD) can be administered to stimulate pancreatic secretion
and improve delineation of the main pancreatic duct or assess for leak during MRCP [92-94]. The manufacturer recommends an
intravenous dose of 0.2 mcg/kg (slowly, over 1 minute) [95].

GASTROINTESTINAL (GI) CONTRAST MEDIA IN ADULTS: INDICATIONS AND GUIDELINES 66
A relative contraindication to secretin administration is acute pancreatitis, as symptoms can be exacerbated [96]. Other side effects
include nausea, flushing, abdominal pain, and vomiting [95]. Allergic-like reactions are rare, with few (if any) reported [97].
Nonetheless, the manufacturer recommends giving a test dose of 0.2 mcg to assess for reactions for 1 minute before giving the
remainder of the dose [95].
Immediate hypersensitivity reactions to enteric contrast media
Nonvascular (enteric or nonenteric) administration of contrast agents results in some systemic absorption of the agent [98]. Studies
suggest that approximately 1% to 2% of enteric iodinated contrast media are normally absorbed and subsequently excreted into
the urinary tract after oral or rectal administration [99,100]. As allergic-like reactions are not considered to be dose related and
can occur with less than 1 ml of IV contrast media, it is generally accepted that allergic-like reactions can occur even from the
small amounts of contrast medium absorbed from the gastrointestinal tract. However, these reactions allergic-like reactions after
nonvascular administration of contrast remain infrequent compared to reactions after intravenous administration [101 -103], with
only very rare reports of moderate or severe allergic-like reactions to orally or rectally administered iodinated contrast media [36].
Use of Gastrografin® and Gastroview® is contraindicated in patients with allergy to salts of diatrizoic acid or other ingredients
of the solution [22,23].
Strategies for Premedication or Agent-Switching
There is variability in the approach to premedication for patients with a history of contrast allergies who are scheduled for exams
involving nonvascular administration of contrast. Some favor following the same guidelines as for vascular administration of contrast
(see “Indications for Premedication” in Chapter 4). Others do not recommend premedication for these patients given the rarity of
immediate hypersensitivity reactions after nonvascular administration [98]. This latter approach has also been adopted by some
outside of radiology, with a study showing that most urologists do not prescribe prophylaxis for contrast allergies prior to
endourologic procedures [104]. Yet another approach is to only premedicate patients with a history of a severe immediate
hypersensitivity reaction to the same class of contrast agent being administered extravascularly (see Table 1 for description of
categories of acute reactions) [105].
It is known that premedication does not prevent all repeat reactions. Another mitigation strategy is to change contrast agents, whether
choosing a different agent in the same class or selecting a different class of agent (iodinated contrast media vs. barium vs. gadolinium-
based agent). Changing contrast media, even within the same class, may be more effective than premedication in preventing rep eat
reaction [98,106-109]. This is also discussed in Chapter 4 of the manual (link to “Changing Contrast Media Within the Same Class”).
Treatment of adverse reactions to GI contrast agents and ancillary drugs
Facilities should be equipped with basic supplies and medications to evaluate and treat adverse reactions to oral contrast agents and
ancillary drugs. Treatment of allergic-like reactions to oral contrast agents can follow the same algorithmic approach as treatment of
reactions to intravenous contrast agents. Algorithms, scenarios, and potential supplies for contrast reaction kits are provided in Table
2, Table 3, and Table 4. Treatment of other non-allergic adverse reactions can vary and should be based on patient symptoms.
References
1. Gelfand DW, Ott DJ. Barium sulfate suspensions: an evaluation of available products. AJR Am J Roentgenol 1982;138:935-41.
2. Laufer I. LM. Double Contrast Gastrointestinal Radiology. 2nd ed. Philadelphia, PA: WB Saunders; 1992.
3. Miller RE SJ. Radiographic Contrast Agents. Baltimore, MD: University Park Press; 1977.
4. Foley MJ, Ghahremani GG, Rogers LF. Reappraisal of contrast media used to detect upper gastrointestinal perforations: comparison of ionic water-
soluble media with barium sulfate. Radiology 1982;144:231-7.
5. Swanson JO, Levine MS, Redfern RO, Rubesin SE. Usefulness of high -density barium for detection of leaks after esophagogastrectomy, total
gastrectomy, and total laryngectomy. AJR Am J Roentgenol 2003;181:415-20.
6. Schuur JD, Chu G, Sucov A. Effect of oral contrast for abdominal computed tomography on emergency department length of stay. Emerg Radiol
2010;17:267-73.
7. Kielar AZ, Patlas MN, Katz DS. Oral contrast for CT in patients with acute non-traumatic abdominal and pelvic pain: what should be its current role?
Emerg Radiol 2016;23:477-81.
8. Levenson RB, Camacho MA, Horn E, Saghir A, McGillicuddy D, Sanchez LD. Eliminating routine oral contrast use for CT in the emergency
department: impact on patient throughput and diagnosis. Emerg Radiol 2012;19:513-7.
9. Garrett PR, Meshkov SL, Perlmutter GS. Oral contrast agents in CT of the abdomen. Radiology 1984;153:545-6.
10. Ott DJ, Gelfand DW. Gastrointestinal contrast agents. Indications, uses, and risks. Jama 1983;249:2380 -4.
11. Vessal K, Montali RJ, Larson SM, Chaffee V, James AE, Jr. Evaluation of barium and gastrografin as contrast media for the diagnosis of esophageal
ruptures or perforations. Am J Roentgenol Radium Ther Nucl Med 1975;123:307-19.
12. Buschmann C, Schulz F, Tsokos M. Fatal aspiration of barium sulfate. Forensic Sci Med Pathol 2011;7:63-4.
13. Boyd EM, Abel M. The acute toxicity of barium sulfate administered intragastrically. Can Med Assoc J 1966;94:849-53.
GASTROINTESTINAL (GI) CONTRAST MEDIA IN ADULTS: INDICATIONS AND GUIDELINES 67
14. Savry C, Bouche O, Lefrant JY, Saissy G, Allain P. [Barium sulfate poisoning?]. Ann Fr Anesth Reanim 1999;18:454 -7.
15. Bowen LN, Subramony SH, Cheng J, Wu SS, Okun MS. Elementary, my dear Dr. Allen: the case of barium toxicity and Pa Ping. Neurology
2010;74:1546-9.
16. Barium toxicity after exposure to contaminated contrast solution--Goias State, Brazil, 2003. MMWR Morb Mortal Wkly Rep 2003;52:1047-8.
17. Pélissier-Alicot AL, Léonetti G, Champsaur P, Allain P, Mauras Y, Botta A. Fatal poisoning due to intravasation after oral administration of barium
sulfate for contrast radiography. Forensic Sci Int 1999;106:109-13.
18. Roza O, Berman LB. The pathophysiology of barium: hypokalemic and cardiovascular effects. J Pharmacol Exp Ther 1971;177:433-9.
19. Ahlawat SK, Sachdev A. Hypokalaemic paralysis. Postgrad Med J 1999;75:193-7.
20. Ghose A, Sayeed AA, Hossain A, Rahman R, Faiz A, Haque G. Mass barium carbonate poisoning with fatal outcome, lessons learned: a case series.
Cases J 2009;2:9327.
21. Payen C, Dellinger A, Pulce C, et al. Intoxication by large amounts of barium nitrate overcome by early massive K supplementation and oral
administration of magnesium sulphate. Hum Exp Toxicol 2011;30:34-7.
22. GUERBET. MD-Gastroview®. Available at: https://www.guerbet.com/en-us/products-solutions/contrast-agents/md-gastroview-diatrizoate-
meglumine-and-diatrizoate-sodium-solution-usp. Accessed September 19, 2023.
23. BRACCO. Gastrografin®. Available at: https://www.bracco.com/en-ca/product/gastrografin. Accessed September 19, 2023.
24. GE HEALTHCARE. Omnipaque™ (iohexol) injection. Available at: https://www.gehealthcare.com/products/contrast-media/omnipaque. Accessed
September 19, 2023.
25. GE HEALTHCARE. OMNIPAQUE. Available at: https://www.gehealthcare.com/-/jssmedia/GEHC/US/Files/Products/Contrast-
Media/Omnipaque/omnipaque-uspi.pdf. Accessed September 19, 2023.
26. Pickhardt PJ. Positive Oral Contrast Material for Abdominal CT: Current Clinical Indications and Areas of Controversy. American Journal of
Roentgenology 2020;215:69-78.
27. Biondo S, Parés D, Mora L, Martí Ragué J, Kreisler E, Jaurrieta E. Randomized clinical study of Gastrografin administration in patients with
adhesive small bowel obstruction. Br J Surg 2003;90:542-6.
28. Burge J, Abbas SM, Roadley G, et al. Randomized controlled trial of Gastrografin in adhesive small bowel obstruction. ANZ J Surg 2005;75:672-4.
29. Kapoor S, Jain G, Sewkani A, Sharma S, Patel K, Varshney S. Prospective evaluation of oral gastrografin in postoperative small bowel obstruction. J
Surg Res 2006;131:256-60.
30. Choi HK, Chu KW, Law WL. Therapeutic value of gastrografin in adhesive small bowel obstruction after unsuccessful conservative treatment: a
prospective randomized trial. Ann Surg 2002;236:1-6.
31. Zer M, Rubin M, Dintsman M. Dissolution of Barium-impaction ileus by Gastrografin. Dis Colon Rectum 1978;21:430-4.
32. Agrons GA, Corse WR, Markowitz RI, Suarez ES, Perry DR. Gastrointestinal manifestations of cystic fibrosis: radiologic-pathologic correlation.
Radiographics 1996;16:871-93.
33. Bracco Imaging Canada. GASTROGRAFIN® - PRODUCT MONOGRAPH INCLUDING PATIENT MEDICATION INFORMATION. Available
at: https://pdf.hres.ca/dpd_pm/00065640.PDF. Accessed September 19, 2023.
34. Halme L, Edgren J, von Smitten K, Linden H. Increased urinary excretion of iohexol after enteral administration in patients with ileal Crohn's disease.
A new test for disease activity. Acta Radiol 1993;34:237-41.
35. Marinelli DL, Mintz MC. Absorption and excretion of dilute gastrografin during computed tomography in pseudomembranous colitis. J Comput
Tomogr 1987;11:236-8.
36. Miller SH. Anaphylactoid reaction after oral administration of diatrizoate meglumine and diatrizoate sodium solution. AJR Am J Roentgenol
1997;168:959-61.
37. Gmeinwieser J, Erhardt W, Reimann HJ, Babic R, Speck U, Wenzel-Hora B. Side effects of water-soluble contrast agents in upper gastrointestinal
tract. Invest Radiol 1990;25 Suppl 1:S27-8.
38. Horton KM, Fishman EK, Gayler B. The use of iohexol as oral contrast for computed tomography of the abdomen and pelvis. J Com put Assist
Tomogr 2008;32:207-9.
39. Seltzer SE, Jones B, McLaughlin GC. Proper choice of contrast agents in emergency gastrointestinal radiology. CRC Crit Rev Di agn Imaging
1979;12:79-99.
40. Gelfand DW. Complications of gastrointestinal radiologic procedures: I. Complications of routine fluoroscopic studies. Gastro intest Radiol
1980;5:293-315.
41. Reich SB. Production of pulmonary edema by aspiration of water-soluble nonabsorbable contrast media. Radiology 1969;92:367-70.
42. Beng CG, Wellby ML, Symons RG, Stuart S, Marshall J. The effects of iopodate on the serum iodothyronine pattern in normal sub jects. Acta
Endocrinol (Copenh) 1980;93:175-8.
43. Kolbe AB, Fletcher JG, Froemming AT, et al. Evaluation of Patient Tolerance and Small-Bowel Distention With a New Small-Bowel Distending
Agent for Enterography. American Journal of Roentgenology 2016;206:994-1002.
44. Ajaj W, Goehde SC, Schneemann H, Ruehm SG, Debatin JF, Lauenstein TC. Oral contrast agents for small bowel MRI: comparison of different
additives to optimize bowel distension. Eur Radiol 2004;14:458-64.
45. Dillman JR, Towbin AJ, Imbus R, Young J, Gates E, Trout AT. Comparison of Two Neutral Oral Contrast Agents in Pediatric Patients: A
Prospective Randomized Study. Radiology 2018;288:245-51.
46. Winter TC, Ager JD, Nghiem HV, Hill RS, Harrison SD, Freeny PC. Upper gastrointestinal tract and abdomen: water as an orally administered
contrast agent for helical CT. Radiology 1996;201:365-70.
47. Koo CW, Shah-Patel LR, Baer JW, Frager DH. Cost-effectiveness and patient tolerance of low-attenuation oral contrast material: milk versus
VoLumen. AJR Am J Roentgenol 2008;190:1307-13.
48. Thompson SE, Raptopoulos V, Sheiman RL, McNicholas MM, Prassopoulos P. Abdominal helical CT: milk as a low-attenuation oral contrast agent.
Radiology 1999;211:870-5.
49. Mattrey RF, Hajek PC, Gylys-Morin VM, et al. Perfluorochemicals as gastrointestinal contrast agents for MR imaging: preliminary studies in rats and
humans. AJR Am J Roentgenol 1987;148:1259-63.
50. Arpurt JP, Caroli-Bosc FX, Harris A, Delmont J. Reflux of air or barium into the biliary ducts. Gastroenterology 1992;103:1989-90.
51. Walsham A, Larsen J. Adverse effects of barium sulfate in the biliary tract. Diagn Interv Radiol 2008;14:94-6.
52. Zeman RK, Burrell MI. Gallbladder and Bile Duct Imaging: A Clinical Radiologic Approach: Churchill Livingstone; 1987.
53. Hanson JA, Borre C, Rivers G. What is your diagnosis? Foreign body obstruction of the duodenum with reflux of barium sulfate into the gallbladder
GASTROINTESTINAL (GI) CONTRAST MEDIA IN ADULTS: INDICATIONS AND GUIDELINES 68
and intrahepatic bile ducts. J Am Vet Med Assoc 1998;213:1257-8.
54. Gibney RG BH. Cholangiography and pancreatography. 2nd ed. Rockville, MD: Aspen Publishers; 1989.
55. J. S. Barium sulfate: toxicity and complications. 2nd ed. Rockville, MD: Aspen Publishers; 1989.
56. Hunter TB FL. Outline of radiographic contrast agents. Appl Radiol 1987:16:137 -58.
57. Sable RA, Rosenthal WS, Siegel J, Ho R, Jankowski RH. Absorption of contrast medium during ERCP. Dig Dis Sci 1983;28:801 -6.
58. Mann K, Rendl J, Busley R, et al. Systemic iodine absorption during endoscopic application of radiographic contrast agents for endoscopic retrograde
cholangiopancreaticography. Eur J Endocrinol 1994;130:498-501.
59. Hopper KD, Wegert SJ, Hallgren SE. Renal excretion of endoscopic retrograde cholangiopancreatography injected contrast. A com mon phenomenon.
Invest Radiol 1989;24:394-6.
60. Draganov P, Cotton PB. Iodinated contrast sensitivity in ERCP. Am J Gastroenterol 2000;95:1398 -401.
61. Draganov PV, Forsmark CE. Prospective evaluation of adverse reactions to iodine-containing contrast media after ERCP. Gastrointest Endosc
2008;68:1098-101.
62. Lawrence C, Cotton PB. Gadolinium as an alternative contrast agent for therapeutic ERCP in the iodine-allergic patient. Endoscopy 2009;41:564-7.
63. Trottier-Tellier F, Harvey L, Bernard H, Baillargeon J. A325 RISK EVALUATION OF ENDOSCOPIC RETROGRADE
CHOLANGIOPANCREATOGRAPHY (ERCP)-RELATED CONTRAST MEDIA (CM) ALLERGIC REACTION AMONG PATIENTS KNOWN
FOR ADVERSE REACTION TO IODINE CONTAINING PRODUCT. Journal of the Canadian Association of Gastroenterology 2018;1:565-66.
64. Nilsson U. Adverse reactions to iotroxate at intravenous cholangiography. A prospective clinical investigation and review of the literature. Acta
Radiol 1987;28:571-5.
65. Persson A, Dahlström N, Smedby O, Brismar TB. Three-dimensional drip infusion CT cholangiography in patients with suspected obstructive biliary
disease: a retrospective analysis of feasibility and adverse reaction to contrast material. BMC Med Imaging 2006;6:1.
66. Schroeder T, Radtke A, Debatin JF, et al. Contrast-enhanced multidetector-CT cholangiography after living donor liver transplantation.
Hepatogastroenterology 2007;54:1176-80.
67. Schroeder T, Radtke A, Kuehl H, Debatin JF, Malagó M, Ruehm SG. Evaluation of living liver donors with an all-inclusive 3D multi-detector row
CT protocol. Radiology 2006;238:900-10.
68. Frydrychowicz A, Lubner MG, Brown JJ, et al. Hepatobiliary MR imaging with gadolinium-based contrast agents. J Magn Reson Imaging
2012;35:492-511.
69. FDA. MultiHance (gadobenate dimeglumine) Injection. Available at:
https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/021357s009lbl.pdf. Accessed September 19, 2023.
70. FDA. EOVIST (Gadoxetate Disodium) Injection for intravenous use Available at:
https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/022090s004lbl.pdf. Accessed September 19, 2023.
71. Dorta G, Uske A, Blum AL. Meglumine gadoterate: a new safe radiocontrast medium for endoscopic retrograde cholangiopancreatography?
Digestion 1997;58:289-92.
72. FDA. GLUCAGON for injection, for subcutaneous, intramuscular or intravenous use Available at:
https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/201849s005lbl.pdf. Accessed September 19, 2023.
73. Bova JG, Jurdi RA, Bennett WF. Antispasmodic drugs to reduce discomfort and colonic spasm during barium enemas: comparison of oral
hyoscyamine, i.v. glucagon, and no drug. AJR Am J Roentgenol 1993;161:965-8.
74. Thoeni RF, Margulis AR. The state of radiographic technique in the examination of the colon: a survey in 1987. Radiology 1988 ;167:7-12.
75. Miller RE, Chernish SM, Brunelle RL. Gastrointestinal radiography with glucagon. Gastrointest Radiol 1979;4:1 -10.
76. Oppenheimer J, Ray CE, Jr., Kondo KL. Miscellaneous pharmaceutical agents in interventional radiology. Semin Intervent Radiol 2010;27:422-30.
77. Skucas J. The use of antispasmodic drugs during barium enemas. AJR Am J Roentgenol 1994;162:1323-5.
78. Chernish SM, Maglinte DD. Glucagon: common untoward reactions--review and recommendations. Radiology 1990;177:145-6.
79. Feczko PJ, Simms SM, Iorio J, Halpert R. Gastroduodenal response to low-dose glucagon. AJR Am J Roentgenol 1983;140:935-40.
80. Skucas J. Optimal dose of glucagon. Radiology 1992;183:325.
81. Chernish SM, Maglinte DD, Brunelle RL. The laboratory response to glucagon dosages used in gastrointestinal examinations. Inv est Radiol
1988;23:847-52.
82. Gutzeit A, Binkert CA, Koh DM, et al. Evaluation of the anti-peristaltic effect of glucagon and hyoscine on the small bowel: comparison of
intravenous and intramuscular drug administration. Eur Radiol 2012;22:1186-94.
83. Morris CH, Baker J. Glucagon. StatPearls. Treasure Island (FL): StatPearls Publishing
Copyright © 2023, StatPearls Publishing LLC.; 2023.
84. Moeller G, Hughes JJ, Mangano FA, Gelormini R, Tomlinson TL, DiPalma JA. Comparison of L-hyoscyamine, glucagon, and placebo for air-
contrast upper gastrointestinal series. Gastrointest Radiol 1992;17:195-8.
85. Bova JG, Bhattacharjee N, Jurdi R, Bennett WF. Comparison of no medication, placebo, and hyoscyamine for reducing pain during a barium enema.
AJR Am J Roentgenol 1999;172:1285-7.
86. Wagner M, Klessen C, Rief M, et al. High-resolution T2-weighted abdominal magnetic resonance imaging using respiratory triggering: impact of
butylscopolamine on image quality. Acta Radiol 2008;49:376-82.
87. Froehlich JM, Daenzer M, von Weymarn C, Erturk SM, Zollikofer CL, Patak MA. Aperistaltic effect of hyoscine N-butylbromide versus glucagon on
the small bowel assessed by magnetic resonance imaging. Eur Radiol 2009;19:1387-93.
88. Ghobrial PM, Neuberger I, Guglielmo FF, et al. Cine MR enterography grading of small bowel peristalsis: evaluation of the ant iperistaltic
effectiveness of sublingual hyoscyamine sulfate. Acad Radiol 2014;21:86-91.
89. Young BM, Fletcher JG, Booya F, et al. Head-to-head comparison of oral contrast agents for cross-sectional enterography: small bowel distention,
timing, and side effects. J Comput Assist Tomogr 2008;32:32-8.
90. Garra G, Singer AJ, Bamber D, Chohan J, Troxell R, Thode HC, Jr. Pretreatment of patients requiring oral contrast abdominal computed tomography
with antiemetics: a randomized controlled trial of efficacy. Ann Emerg Med 2009;53:528-33.
91. Sewell DD, Kodsi AB, Caligiuri MP, Jeste DV. Metoclopramide and tardive dyskinesia. Biol Psychiatry 1994;36:630 -2.
92. Matos C, Winant C, Devière J. Magnetic resonance pancreatography. Abdom Imaging 2001;26:243 -53.
93. Dreiling DA, Messer J. The secretin story: a saga in clinical medicine and gastrointestinal physiology. Am J Gastroenterol 1978;70:455-79.
94. Laugier R. Dynamic endoscopic manometry of the response to secretin in patients with chronic pancreatitis. Endoscopy 1994;26:222-7.
95. FDA. ChiRhoStim® (Human Secretin) Injection, lyophilized powder for intravenous use, 16 mcg and 40 mcg vials Available at:
GASTROINTESTINAL (GI) CONTRAST MEDIA IN ADULTS: INDICATIONS AND GUIDELINES 69
https://www.accessdata.fda.gov/drugsatfda_docs/label/2007/021256s002lbl.pdf. Accessed September 19, 2023.
96. Boraschi P, Donati F, Gigoni R, et al. Pancreatic transplants: secretin-stimulated MR pancreatography. Abdom Imaging 2007;32:207-14.
97. Wu B, Conwell DL. The endoscopic pancreatic function test. Am J Gastroenterol 2009;104:2381-3.
98. Davis PL. Anaphylactoid reactions to the nonvascular administration of water-soluble iodinated contrast media. AJR Am J Roentgenol
2015;204:1140-5.
99. Eisenberg RL, Hedgcock MW, Shanser JD, Brenner RJ, Gedgaudas RK, Marks WM. Iodine absorption from the gastrointestinal tract during
hypaque-enema examination. Radiology 1979;133:597-9.
100. Rosen RS, Jacobson G. VISIBLE URINARY TRACT EXCRETION FOLLOWING ORAL ADMINISTRATION OF WATER-SOLUBLE
CONTRAST MEDIA. Radiology 1965;84:1031-2.
101. Kim YS, Choi YH, Cho YJ, et al. Incidence of Breakthrough Reaction in Patients with Prior Acute Allergic-Like Reactions to Iodinated Contrast
Media according to the Administration Route. Korean J Radiol 2018;19:352-57.
102. Joseph JP, Domino P, Bird VG, Sharma N, Ford S, Caruso LJ. Outcomes in Patients with Known Contrast Allergy Undergoing Contrast-Enhanced
Endourologic Procedures: A Retrospective Cohort Study. J Endourol 2021;35:1857-62.
103. Widmark JM. Imaging-related medications: a class overview. Proc (Bayl Univ Med Cent) 2007;20:408 -17.
104. Mohapatra A, Hyun G, Semins MJ. Trends in the Usage of Contrast Allergy Prophylaxis for Endourologic Procedures. Urology 2019 ;131:53-56.
105. Yale School of Medicine. Premedication Policy for Non-Vascular Administration of Contrast in Patients with Contrast Allergy.
Availableat:https://medicine.yale.edu/diagnosticradiology/qualitysafety/nonvascular%20contrast%20v4_442936_51324_v1_456180_51324_v1.pdf.
Accessed September 19, 2023.
106. Umakoshi H, Nihashi T, Takada A, et al. Iodinated Contrast Media Substitution to Prevent Recurrent Hypersensitivity Reactions: A Systematic
Review and Meta-Analysis. Radiology 2022;305:341-49.
107. McDonald JS, Larson NB, Kolbe AB, et al. Prevention of Allergic-like Reactions at Repeat CT: Steroid Pretreatment versus Contrast Material
Substitution. Radiology 2021;301:133-40.
108. Abe S, Fukuda H, Tobe K, Ibukuro K. Protective effect against repeat adverse reactions to iodinated contrast medium: Premedication vs. changing the
contrast medium. Eur Radiol 2016;26:2148-54.
109. Park SJ, Kang DY, Sohn KH, et al. Immediate Mild Reactions to CT with Iodinated Contrast Media: Strategy of Contrast Media Readministration
without Corticosteroids. Radiology 2018;288:710-16.
Revision History
2013: Revision
2024: Major Revision
ACR–ASNR POSITION STATEMENT ON THE USE OF GADOLINIUM CONTRAST AGENTS 70
ACR–ASNR POSITION STATEMENT ON THE USE OF
GADOLINIUM CONTRAST AGENTS
Following U.S. Food and Drug Administration (FDA) approval in 1988, gadolinium-based contrast agents (GBCAs) have been
used for diagnosis and treatment guidance in more than 300 million patients worldwide. GBCAs increase the conspicuity of
diseased tissues. All GBCAs share a common structure of an organic ligand that tightly binds to and improves the stability,
solubility, and safety of the central gadolinium heavy metal ion. In typical patients, the chelate is mostly eliminated via the
kidneys, with some amount of liver excretion demonstrated for a few of the agents.
Since 2006, radiologists have withheld some GBCAs from patients with acute kidney injury and/or severe chronic kidney
disease, if the estimated glomerular filtration rate (GFR) is <30 mL/min/1.73 m
2
, because of the increased risk of nephrogenic
systemic fibrosis (NSF). NSF is a rare but serious systemic disease characterized by fibrosis of the skin and other tissues
throughout the body in renally impaired individuals. As a result of judicious use of GBCAs among patients with compromised
renal function and a decrease in utilization of those GBCAs that are more highly associated with NSF, there has been a drastic
reduction in the number of cases encountered since restrictive guidelines were put into place after the association of NSF with
GBCAs was identified in 2006.
Recently, residual gadolinium has been found within the brain tissue of patients who received multiple doses of GBCAs over
their lifetimes. For reasons that remain unclear, gadolinium deposition appears to occur preferentially in certain specific a reas
of the brain, even in the absence of clinically evident disease and in the setting of an intact blood brain barrier. Such deposition
is not expected, and led the FDA to publish a Safety Alert in July of 2015 indicating that they were actively investigating the
risk and clinical significance of these gadolinium deposits. To date, no adverse health effects have been uncovered, but the
radiology community has initiated a rigorous investigation.
Gadolinium deposition in the brain may be dose dependent and can occur in patients with no clinical evidence of kidney or
liver disease. Fortunately, there have been no reports to date to suggest these deposits are associated with histologic changes
that would suggest neurotoxicity, even among GBCAs with the highest rates of deposition. Although there are no known adverse
clinical consequences associated with gadolinium deposition in the brain, additional research is warranted to elucidate the
mechanisms of deposition, the chelation state of these deposits, the relationship to GBCA stability and binding affinity, and
theoretical toxic potential, which may be different for different GBCAs. Until we fully understand the mechanisms involved
and their clinical consequences, the safety and tissue deposition potential of all GBCAs must be carefully evaluated.
GBCAs provide crucial, life-saving medical information. Each time a gadolinium-enhanced MRI study is considered, it would
be prudent to consider the clinical benefit of the diagnostic information or treatment result that MRI or MRA may provide
against the unknown potential risk of gadolinium deposition in the brain for each individual patient. Particular attention should
be paid to pediatric and other patients who may receive many GBCA-enhanced MRI studies over the course of their lifetimes.
If the decision for an individual patient is made to use a GBCA for an MRI study, multiple factors need to be considered when
selecting a GBCA, including diagnostic efficacy, relaxivity, rate of adverse reactions, dosing/concentration, and propensity to
deposit in more sensitive organs such as the brain. As this gadolinium deposition phenomenon remains a relatively undefined
clinical phenomenon, and accurate and complete data may be useful as investigations proceed, the identity and dose of GBCA
used should be recorded after each intravenous administration.
The radiology community will continue to assess the safety of GBCAs and modify clinical practice recommendations
accordingly as new data becomes available.
ADVERSE REACTIONS TO GADOLINIUM-BASED CONTRAST MEDIA 71
ADVERSE REACTIONS TO GADOLINIUM-BASED CONTRAST MEDIA
Gadolinium-based contrast media (GBCM) have been approved for parenteral use since the late 1980s. These agents can be
differentiated on the basis of chelate chemistry, stability, viscosity, osmolality, and, in some cases, effectiveness for specific
applications. GBCM are extremely well tolerated by the vast majority of patients in whom they are injected. Acute adverse reactions
are encountered with a lower frequency than is observed after administration of iodinated contrast media.
Adverse Reactions
The adverse event rate for GBCM administered at clinical doses (0.1–0.2 mmol/kg for most GBCM) ranges from 0.07% to 2.4%.
Most reactions are mild and physiologic, including coldness, warmth, or pain at the injection site; nausea with or without vomiting;
headache; paresthesias; and dizziness. Allergic- like reactions are uncommon and vary in frequency from 0.004% to –0.7%. The
manifestations of an allergic- like reaction to a GBCM are similar to those of an allergic-like reaction to an iodinated contrast
medium. Severe life-threatening anaphylactic reactions occur [1-6] but are exceedingly rare (0.001% to 0.01%) [7-9] In an
accumulated series of 687,000 doses there were only five severe reactions [10]. In a survey of 20 million administered doses, there
were 55 severe reactions. A large single-institution study that included more than 100,000 GBCM injections demonstrated an
allergic-like reaction frequency of 0.15%, with 0.13% mild reactions and 0.006% severe reactions (six reactions) [11]. Fatal
reactions to gadolinium chelate agents occur but are extremely rare [12].
GBCM administered to patients with acute kidney injury or severe chronic kidney disease can result in a syndrome of nephrogenic
systemic fibrosis (NSF) [13,14]. For more information, see the chapter on Nephrogenic Systemic Fibrosis. GBCM are not considered
nephrotoxic at dosages approved for MR imaging.
Risk Factors
The frequency of acute adverse reactions to GBCM is about eight times higher in patients with a previous reaction to GBCM. At
many institutions, a prior allergic-like reaction to GBCM is often an indication for corticosteroid prophylaxis prior to subsequent
exposures. One GBCM, gadobenate dimeglumine, has FDA labeling contraindicating use in patients who have a history of an
allergic-like reaction to GBCM. Some reports have suggested that GBCM that have been most commonly associated with NSF are
less likely to be associated with allergic-like reactions and vice versa [15].
Patients with asthma and various other allergies may have a mild increased risk for an allergic-like reaction to GBCM compared to
the general population, but many institutions do not have special procedures for these patients given the extremely low overall
reaction rate for GBCM. There is no cross-reactivity between GBCM and iodinated contrast media.
In a patient with previous moderate or severe allergic-like reactions to a specific GBCM, it may be prudent to use a different GBCM
and premedicate for subsequent MR examinations, although there are no published studies to confirm that this approach is
efficacious in reducing the likelihood of a repeat contrast reaction.
The Safety of Gadolinium-Based Contrast Media in Patients with Sickle Cell Disease
Early in vitro research investigating the effects of a strong external magnetic field (e.g., MR magnet) on red blood cells (erythrocytes)
suggested that fully deoxygenated sickle erythrocytes align perpendicularly to a magnetic field. It was hypothesized that this
alignment could further restrict sickle erythrocyte flow through small vessels and promote vaso-occlusive complications in sickle
cell patients [16]. Based on this supposition, FDA package inserts suggested caution in patients with sickle cell disease for two
GBCM approved for use in the United States (gadoversetamide [OptiMARK, Guerbet] and gadoteridol [Prohance, Bracco
Diagnostics]).
To the best of our knowledge and noted in a review of the literature [17], there has been no documented in vivo vaso-occlusive or
hemolytic complication directly related to the IV administration of GBCM in a sickle cell disease patient. A small retrospective
study with a control group showed no significantly increased risk of vaso- occlusive or hemolytic adverse events when administering
GBCM to sickle cell disease patients [18]. Additionally, several small scientific studies [19-21] of patients with sickle cell disease
have employed MR imaging with GBCM without reported adverse effects.
Therefore, the risk to patients with sickle cell disease from IV-administered GBCM at approved dosages is very low or nonexistent,
and there is no reason to withhold these agents from these patients when their use is otherwise indicated.
ADVERSE REACTIONS TO GADOLINIUM-BASED CONTRAST MEDIA 72
Breath-holding Difficulty with Gadoxetate Disodium
Several studies have noted that gadoxetate disodium may be associated with transient severe respiratory motion- related artifact that
manifests in the arterial phase of dynamic T1-weighted gradient echo imaging and resolves shortly thereafter [22-26]. This
manifestation has been described as “transient dyspnea”. At one institution, patient surveys showed that significantly more p atients
complained of subjective shortness of breath following gadoxetate disodium compared to gadobenate dimeglumine exposure [22].
The reported rate of occurrence of “transient dyspnea” has varied by site, imaging acquisition parameters, and administered volume,
ranging from 4% to 14% [22-26].
Based on the volume-effect relationship and the lack of identifiable atopic covariates, this appears to be a physiologic reaction,
manifesting as dyspnea or breath-holding difficulty that is unique to this agent [25]. The event is self-limited and does not appear to
relate to allergic-like bronchospasm [22,24,25]. Therefore, corticosteroid prophylaxis is unlikely to be beneficial and is not felt to
be indicated. Strong risk factors include a larger administered volume irrespective of patient weight (20 mL doses are twice as likely
to cause the artifact as 10 mL doses) [25], chronic obstructive pulmonary disease (patients with COPD have a 35–40% event rate)
[25], and re-administering the agent to patients who have previously had a similar reaction (previously affected patients have a 60%
event rate on subsequent studies compared to a 5% event rate in the unaffected population) [26]. Imaging strategies to avoid the
artifact include minimizing the injected volume (≤10 mL), avoiding the agent in patients who have experienced it before, and
acquiring more than one arterial phase with a short temporal footprint [22-26]
Treatment of Acute Adverse Reactions
Treatment of acute adverse reactions to GBCM is similar to that for acute reactions to iodinated contrast media (see Tables 2 and
3). In any facility where contrast media are injected, it is imperative that personnel trained in recognizing and handling reactions
and the equipment and medications to do so be on site or immediately available. Most MR facilities take the position that pa tients
requiring treatment should be taken out of the imaging room immediately and away from the magnet so that none of the resuscitative
equipment becomes a magnetic hazard.
Extravasation
Extravasation events to GBCM are rare, with one series demonstrating a rate of 0.05% (28,000 doses). Laboratory studies in animals
have demonstrated that both gadopentetate dimeglumine and gadoteridol are less toxic to the skin and subcutaneous tissues than are
equal volumes of iodinated contrast media [27,28]. The small volumes typically injected for MR studies limit the chances of
developing compartment syndrome. For these reasons the likelihood of a significant injury resulting from extravasated MR contrast
media is extremely low.
Serum Calcium Determinations
Some linear nonionic GBCM (e.g., gadoversetamide, gadodiamide) may interfere with total serum calcium values as determined
with some calcium assay methods [29,30]. These GBCM do not cause actual reductions in serum calcium. Rather, they interfere
with the test, leading to falsely low serum calcium laboratory values. In one report by Brown and associates [30], calcium levels
measured by only one of three different assays (the orthocresolphthalein assay) showed a temporary decrease for just two of four
studied GBCM (gadopentetate and gadoteridol had no effect), the length and severity of which closely mirrored the concentration
of the measured GBCM in blood.
Off-Label Use of MRI Contrast Agents
In the past, radiologists often used GBCM in an off-label fashion (e.g., off-label higher doses or off-label indications). By definition,
such usage is not approved by the FDA. However, physicians have some latitude in off-label GBCM use as guided by clinical
circumstances as long as they can justify such usage in individual cases. Extremely high doses of GBCM much greater than FDA
labeling (which were used frequently in the past) have largely been abandoned, especially in patients with severe chronic kidney
disease and acute kidney injury due to concerns regarding nephrogenic systemic fibrosis.
References
1.
Weiss KL. Severe anaphylactoid reaction after iv. Gd-DTPA. Magn Reson Imaging. 1990;8(6):817-818.
2.
Omohundro JE, Elderbrook MK, Ringer TV. Laryngospasm after administration of gadopentetate dimeglumine. J Magn Reson Imaging.
1992;2(6):729-730.
3.
Tardy B, Guy C, Barral G, Page Y, Ollagnier M, Bertrand JC. Anaphylactic shock induced by intravenous gadopentetate dimeglumine.
Lancet. 1992;339(8791):494.
4.
Takebayashi S, Sugiyama M, Nagase M, Matsubara S. Severe adverse reaction to iv gadopentetate dimeglumine. AJR Am J Roentgenol.
1993;160(3):659.
5.
Witte RJ AL. Life-threatening anaphylactoid reaction after intravenous gadoteridol administration in a patient who had previously received
ADVERSE REACTIONS TO GADOLINIUM-BASED CONTRAST MEDIA 73
gadopentetate dimeglumine. AJNR Am J Neuroradiol. 1994; 15:523-524.
6.
Murphy KJ, Brunberg JA, Cohan RH. Adverse reactions to gadolinium contrast media: a review of 36 cases. AJR Am J Roentgenol.
1996;167(4):847-849.
7.
Murphy KJ BJ, Cohan RH. Adverse reactions to gadolinium contrast media: a review of 36 cases. 1996; 167:847-849.
8.
Runge VM. Safety of approved MR contrast media for intravenous injection. J Magn Reson Imaging. 2000;12(2):205-213.
9.
Runge VM. Safety of magnetic resonance contrast media. Top Magn Reson Imaging. 2001;12(4):309-314.
10.
Murphy KP SK, Cohan RH, Mermillod B, Ellis JH. Occurrence of adverse reactions to gadolinium-based contrast material and management of patients
at increased risk: a survey of the American Society of Neuroradiology Fellowship Directors. Acad Radiol. 1999; 6:656-664.
11.
Davenport MS DJ, Cohan RH, Hussain HK, Khalatbari S, McHugh JB, Ellis JH. Effect of abrupt substitution of gadobenate dimeglu mine for
gadopentetate dimeglumine on rate of allergic-like reactions. Radiology. 2013; 266:773-782.
12.
Jordan RM, Mintz RD. Fatal reaction to gadopentetate dimeglumine. AJR Am J Roentgenol. 1995;164(3):743-744.
13.
Kanal E, Barkovich AJ, Bell C, et al. ACR guidance document for safe MR practices: 2007. AJR Am J Roentgenol. 2007;188(6):1447-1474.
14.
Kuo PH, Kanal E, Abu-Alfa AK, Cowper SE. Gadolinium-based MR contrast agents and nephrogenic systemic fibrosis. Radiology. 2007;242(3):647-
649.
15.
Jung JW, Kang HR, Kim MH, et al. Immediate hypersensitivity reaction to gadolinium-based MR contrast media. Radiology. 2012;264(2):414-422.
16.
Brody AS, Sorette MP, Gooding CA, et al. AUR memorial Award. Induced alignment of flowing sickle erythrocytes in a magnetic f ield. A preliminary
report. Invest Radiol. 1985;20(6):560-566.
17.
Kanal E, Shellock FG, Talagala L. Safety considerations in MR imaging. Radiology. 1990;176(3):593-606.
18.
Dillman JR, Ellis JH, Cohan RH, et al. Safety of gadolinium-based contrast material in sickle cell disease. J Magn Reson Imaging. 2011;34(4):917-
920.
19.
Umans H, Haramati N, Flusser G. The diagnostic role of gadolinium enhanced MRI in distinguishing between acute medullary bone infarct and
osteomyelitis. Magn Reson Imaging. 2000;18(3):255-262.
20.
Westwood MA, Shah F, Anderson LJ, et al. Myocardial tissue characterization and the role of chronic anemia in sickle cell cardiomyopathy. J Magn
Reson Imaging. 2007;26(3):564-568.
21.
Zimmerman RA. MRI/MRA evaluation of sickle cell disease of the brain. Pediatr Radiol. 2005;35(3):249-257.
22.
Davenport MS, Viglianti BL, Al-Hawary MM, et al. Comparison of acute transient dyspnea after intravenous administration of gadoxetate disodium
and gadobenate dimeglumine: effect on arterial phase image quality. Radiology. 2013;266(2):452- 461.
23.
Pietryga JA, Burke LM, Marin D, Jaffe TA, Bashir MR. Respiratory motion artifact affecting hepatic arterial phase imaging with gadoxetate disodium:
examination recovery with a multiple arterial phase acquisition. Radiology. 2014;271(2):426-434.
24.
Davenport MS CE, Kaza RK, et al. Matched within-patient cohort study of transient arterial-phase respiratory motion related artifact in MRI of the
liver: Gd-EOB-DTPA vs. Gd-BOPTA. Radiology. 2014; 272:123-131.
25.
Davenport MS BM, Pietryga JA, et al. Dose-toxicity relationship of gadoxetate disodium and transient post-injection dyspnea.
AJR Am J Roentgenol. 2014; In press.
26.
Bashir MR CP, Davenport MS, et al. Respiratory motion artifact affecting hepatic arterial phase imaging with gadoxetate disod ium is more common
in patients with a prior episode of arterial phase motion associated with gadoxetate disodium. Radiology. 2015;274: 141-148.
27.
McAlister WH, McAlister VI, Kissane JM. The effect of Gd-dimeglumine on subcutaneous tissues: a study with rats. AJNR Am J Neuroradiol.
1990;11(2):325-327.
28.
Cohan RH, Leder RA, Herzberg AJ, et al. Extravascular toxicity of two magnetic resonance contrast agents. Preliminary experience in the rat. Invest
Radiol. 1991;26(3):224-226.
29.
Lin J IJ, Port M, et al. Interference of magnetic resonance imaging contrast agents with the serum calcium measurement technique using colorimetric
reagents. J Pharm Biomed Anal. 1999; 21:931-943.
30.
Brown JJ, Hynes MR, Wible JH, Jr. Measurement of serum calcium concentration after administration of four gadolinium - based contrast agents to
human volunteers. AJR Am J Roentgenol. 2007;189(6):1539-1544.
Revision History
2 June 2014: Major revisions
15 April 2013: Minor revisions
26 June 2012: Minor revisions May
2008: First version

ADVERSE REACTIONS TO GADOLINIUM-BASED CONTRAST MEDIA 74
Gadolinium Pregnancy Screening Statement
It has been shown that some gadolinium-based contrast agents (GBCAs) pass the placental barrier into the fetal circulation of
nonhuman primates [1]. While multiple small sample size studies have not shown convincing evidence of adverse effects from fetal
exposure to GBCAs [2,3], a 2016 retrospective study cited an increased risk of stillbirth/neonatal death as well as increased risk of
rheumatologic, inflammatory, or infiltrative skin conditions in the offspring after GBCA exposure during pregnancy [4]. While,
questions have been raised regarding study methodology, and these results have not been independently confirmed, both uncertainty
and an abundance of caution in general about the effect of GBCA exposure and retention on the developing fetus has led to statements
in the ACR Manual on Contrast Media [5] and the ACR Manual on MR Safety [6] recommending avoidance of routine administration
of GBCAs to pregnant patients. A decision to administer GBCAs to a pregnant woman should only be made when there is the potential
for significant clinical benefit that outweighs the unknown risk of fetal exposure and should be the product of discussion that involves
the referring provider and patient.
A 2019 study cited increased prevalence of GBCA administration during the first trimester as opposed to later in pregnancy, indicating
that many exposures have occurred without pregnancy screening and/or prior to recognition of pregnancy. This suggests a potential
need for more vigilant pregnancy screening protocols [7]. The current standard of practice is to avoid routine GBCA administration
during pregnancy due to the unknown risk of fetal exposure [5,6,8]; and we recommend that imaging facilities have an established
standardized system of screening in place that includes screening for unsuspected pregnancy prior to GBCA administration within
existing institutional protocols that similarly screen patients prior to exposure to ionizing radiation and/or anesthesia. Protocols
regarding pregnancy testing and reporting of results for pediatric patients and patients with legal guardians must be in accordance
with local and state regulatory statutes.
There is variability in the accuracy of pregnancy tests early in gestation, and at a minimum, testing will be falsely negative in the first
two weeks of pregnancy. As such, there is no screening method that will be 100% effective in detection of unsuspected pregnancy.
Regardless of which screening option is chosen, women of child-bearing age should be informed of the lack of certainty regarding
risk of fetal GBCA exposure. An increased awareness of the issue by the patient may facilitate information sharing between patient
and MRI staff regarding potential for pregnancy that would improve accuracy of screening. Any discussion with referring providers
or patients acknowledging uncertain risks of GBCAs should always be coupled with an assessment of the known diagnostic benefi ts
accrued from contrast-enhanced examinations on a per patient basis.
References
1.
Oh KY, Roberts VH, Schabel MC, Grove KL, Woods M, Frias AE. Gadolinium Chelate Contrast Material in Pregnancy: Fetal Biodistribu tion in the
Nonhuman Primate. Radiology 2015;276:110-8.
2.
De Santis M, Straface G, Cavaliere AF, Carducci B, Caruso A. Gadolinium periconceptional exposure: pregnancy and neonatal out come. Acta
obstetricia et gynecologica Scandinavica 2007;86:99-101.
3.
Spencer JA, Tomlinson AJ, Weston MJ, Lloyd SN. Early report: comparison of breath-hold MR excretory urography, Doppler ultrasound and isotope
renography in evaluation of symptomatic hydronephrosis in pregnancy. Clinical radiology 2000;55:446-53.
4.
Ray JG, Vermeulen MJ, Bharatha A, Montanera WJ, Park AL. Association Between MRI Exposure During Pregnancy and Fetal and Childhood
Outcomes. Jama 2016;316:952-61.
5.
American College of Radiology. ACR Manual on Contrast Media. Available at: https://www.acr.org/-/media/ACR/Files/Clinical-
Resources/Contrast_Media.pdf. Accessed January 31, 2022.
6.
American College of Radiology; ACR Committee on MR Safety. ACR Manual on MR Safety. Available at: https://www.acr.org/-
/media/ACR/Files/Radiology-Safety/MR-Safety/Manual-on-MR-Safety.pdf. Accessed September 7, 2021.
7.
Bird ST, Gelperin K, Sahin L, et al. First-Trimester Exposure to Gadolinium-based Contrast Agents: A Utilization Study of 4.6 Million U.S.
Pregnancies. Radiology 2019;293:193-200.
8.
Committee on Obstetric Practice. Committee Opinion No. 723: Guidelines for Diagnostic Imaging During Pregnancy and Lactation. Obstetrics and
gynecology 2017;130:e210-e16.

ADVERSE REACTIONS TO GADOLINIUM-BASED CONTRAST MEDIA 75
NEPHROGENIC SYSTEMIC FIBROSIS
Definition
Nephrogenic systemic fibrosis (NSF) is a disease, primarily involving the skin and subcutaneous tissues but also known to involve
other organs, such as the lungs, esophagus, heart, and skeletal muscles. Initial symptoms typically include skin thickening and/or
pruritus. Symptoms and signs may develop and progress rapidly, with some affected patients developing contractures and joint
immobility. In some patients, the disease may be fatal.
Associations
Gadolinium-based contrast agent (GBCA) administration
When first described in 2000, NSF was noted to occur predominantly in patients with end-stage chronic kidney disease (CKD),
particularly in patients on dialysis. In 2006, several groups noted a strong association between gadolinium-based contrast agent
(GBCA) administration in patients with advanced renal disease and the development of NSF [1,2], and it is now generally accepted
that GBCA exposure is a necessary factor in the development of NSF, although in rare instances NSF can be diagnosed without
known GBCA exposure. The time between injection of GBCA and the onset of NSF symptoms occurs within days to months in the
vast majority of patients [1-6]; however, in rare cases, symptoms have appeared years after the last reported exposure [5].
The association between NSF development and exposure to GBCAs is widely accepted. It is now known that there are differences in
the likelihood of a patient developing NSF after exposure to different formulations of GBCAs. Almost all unconfounded cases have
been reported after exposure to gadodiamide, gadopentetate dimeglumine, and/or gadoversetamide, while some GBCAs have been
associated with few, if any, confirmed unconfounded cases of NSF. If the prevailing hypothesis is true—that the development of NSF
is related to the release of gadolinium from the chelates that constitute GBCAs—the differences in number of reported cases may, in
part, be explained by differences in the chemical properties of different GBCAs. However, it remains possible that a combination of
other factors, including market share, number of years that the agent has been in use, patient populations, and possible reporting bias,
may have contributed, in part, to some of the differences in the number of reported cases associated with the various GBCAs.
Utilizing both empirical data and theoretical lines of reasoning, the ACR Committee on Drugs and Contrast Media, the European
Medicines Agency (EMA), and the U.S. Food and Drug Administration (FDA) all have classified GBCAs into different groups based
on reported associations with NSF in vulnerable patients, although the scheme used by each is not identical [7,8]. The ACR
classification is given in Table 1.
Chronic kidney disease
Based on current knowledge, it is estimated that patients with end-stage CKD (CKD5, eGFR <15 ml/ min/1.73 m2) and severe CKD
(CKD4, eGFR 15-29 ml/min/1.73 m2) have a 1% to 7% chance of developing NSF after one or more exposures to group 1 GBCAs
with the strongest association with NSF [1-6,9].
However, most patients who developed NSF had end-stage kidney disease and were on dialysis at the time of exposure. Moreover,
among patients with severe CKD (CKD4) that developed NSF (approximately 3% of all reported NSF cases), most had an eGFR
closer to 15 ml/min/1.73 m2 than to 30 ml/min/1.73 m2. There have been rare, published case reports of patients with eGFR values
above 30 ml/min/1.73 m2 [10-12].
Acute kidney injury (AKI)
Between 12% and 20% of confirmed cases of NSF have occurred in patients with AKI, often superimposed upon CKD [13,14]. Some
cases of NSF have developed in patients with AKI without underlying CKD [15]. Hence, AKI alone is also a risk factor for NSF.
High-dose and multiple exposures
Cases of NSF have occurred following a single exposure to a GBCA, including a single exposure to a standard (0.1 mmol/kg) sin gle
dose [5,16]. Nevertheless, NSF occurs most commonly in patients who have received high doses of GBCA, either as a single
administration or cumulatively, in multiple administrations over months to years [6,17].
Most patients with severe CKD exposed to high doses and/or many doses of GBCAs have not developed NSF [5]. One study [18]
described 30 patients who had an eGFR of under 30 ml/min/1.73 m2 and who were exposed to high doses of gadodiamide (median
dose of 90 ml and range of 40 to 200 ml). Only one of the 30 patients subsequently developed NSF, an observed incidence of about
3%.

ADVERSE REACTIONS TO GADOLINIUM-BASED CONTRAST MEDIA 76
A few cases of NSF also have been reported in patients with no known GBCA exposure [19]. In some of these cases, subsequent
tissue biopsy evaluation revealed elevated gadolinium levels in the tissues of these patients, suggesting that at least some of these
patients had prior unknown GBCA exposure [20].
Other possible risk factors
It is not understood why some patients with severe CKD or AKI develop NSF following exposure to GBCAs and others do not, but
a number of possible co-factors have been postulated to play a role. These include metabolic acidosis or medications that predispose
patients to acidosis [1,21]; elevated iron, calcium, and/or phosphate levels [21,22]; high-dose erythropoietin therapy [13];
immunosuppression [6]; vasculopathy [23]; and infection [24] or other acute proinflammatory events [4,25]. However, none of these
have been consistently confirmed as true cofactors. As a result, routine screening for these factors prior to GBCA administration is
not recommended.
Hepatic insufficiency/hepatorenal syndrome
Initially, a number of researchers observed that a disproportionate number of affected patients had concomitant severe liver and renal
dysfunction [4,5], prompting the FDA to warn against the use of GBCAs in patients with “…acute renal insufficiency of any severity
due to the hepatorenal syndrome or in the perioperative liver transplantation period” [26]. However, most data do not support this
conclusion. For example, in one study, a review of the literature found that of 291 patients with NSF, 34 (12%) had concomitant liver
disease [27]; however, all but one of these patients also had known severe renal insufficiency (eGFR of <30 ml/min/1.73 m2) prior
to GBCA administration. Thus, hepatic disease in and of itself, in the absence of AKI or severe CKD, is no longer considered an
independent risk factor for NSF.
Postulated Mechanism
The exact mechanism of NSF causation is unknown. The most widely held hypothesis is that gadolinium ions dissociate from the
chelates in GBCAs in patients with significantly degraded renal function due to the prolonged clearance times of the GBCAs, as well
as to other metabolic factors associated with this level of renal disease. The free gadolinium then binds with an anion such as
phosphate, and the resulting insoluble precipitate is deposited in various tissues [9,28]. A fibrotic reaction ensues, involving the
activation of circulating fibrocytes [28,29]. This hypothesis is supported by the greater presence of gadolinium in affected tissues of
NSF patients relative to unaffected tissues [30]. Nevertheless, the detection of gadolinium in tissues is complicated and is not
considered a requirement for the diagnosis of NSF.
If the propensity for gadolinium to dissociate from various chelates is eventually proved to contribute to, or be primarily responsible
for, the development of NSF, this may help explain, at least in part, why the various GBCAs differ in their apparent NSF safety
profile in at-risk patients, since these agents have varying degrees of stability in vitro and in vivo [31].
Assessment of Risk (See Table 1 for the classification of GBCAs)
Group II agents
Based on the most recent scientific and clinical evidence [32-39] the ACR Committee on Drugs and Contrast Media considers the
risk of NSF among patients exposed to standard or lower than standard doses of group II GBCAs is sufficiently low or possibly
nonexistent such that assessment of renal function with a questionnaire or laboratory testing is optional prior to intravenous
administration. As in all instances, group II GBCAs should only be administered if they are deemed necessary by the supervising
radiologist, and the lowest dose needed for diagnosis should be used as deemed necessary by the supervising radiologist.
1
1
The ACR Committee on Drugs and Contrast Media recognizes that as of this writing (4-6-2017), U.S. Food and Drug Administration (FDA) guidelines and drug
labeling for GBCA have the same recommendations for each GBCA with respect to assessing renal function prior to GBCA administration. Nevertheless, the
committee authoring this Manual has reviewed the evidence and believes that the prevailing weight of clinical evidence on this matter allows less stringent yet
safe patient management which should reduce patient cost and inconvenience. This footnote is designed to alert readers that the ACR recommendations differ in
case their personal philosophy or institutional policies necessitate adherence to the more restrictive FDA guidelines.
Group I and III agents
The ACR Committee on Drugs and Contrast Media concludes that patients receiving group I GBCAs should be considered at risk of
developing NSF if any of the following conditions apply to the patient:
•
On dialysis (of any form)
•
Severe or end-stage CKD (CKD 4 or 5, eGFR < 30 mL / min/1.73 m2) without dialysis
•
AKI [40,41]

ADVERSE REACTIONS TO GADOLINIUM-BASED CONTRAST MEDIA 77
Thus, it is important to identify patients at risk of developing NSF, as defined above, prior to injection of group I and III GBCAs.
The method used to identify such patients may differ for outpatients versus inpatients.
Identifying at-risk outpatients
Outpatients who may be receiving group I or group III agents should be screened for conditions and other factors that may be
associated with renal function impairment.
Simply asking patients if they have a problem with their kidneys is not considered an effective screening tool, as this has been shown
to fail to detect the majority of patients with chronic kidney disease, even those with eGFR<30 ml/min/1.73 m2 [42].
A more reliable method to identify outpatients who may have renal function impairment is to utilize a panel of questions that includes
risk factors for compromised renal function. The following list of risk factors can be used to identify patients who have impaired
renal function. This list represents a blend of published data [43,44] and expert opinion; alternative lists may be as or more effective
depending on practice patterns:
•
History of renal disease, including:
o
Dialysis
o
Kidney transplant
o
Single kidney
o
Kidney surgery
o
History of known cancer involving the kidney(s)
o
History of CKD or prior history of AKI
•
History of diabetes mellitus (optional)
Many additional factors may have deleterious effects on renal function, including multiple myeloma, systemic lupus erythematosus,
urinary tract infection, and use of some medications (e.g., nonsteroidal anti- inflammatory drugs, diuretics, aminoglycosides,
cyclosporine A, amphotericin, and others); however, the ACR Committee on Drugs and Contrast Media currently does not
recommend routinely screening for these additional possible risk factors, since the incremental benefit in patient safety fro m such
screening has not been established and is considered to be low by the committee.
Once an outpatient is identified as being at risk for having reduced renal function based on screening, and group I or group III GBCA
administration is contemplated, renal function should be assessed by laboratory testing (checking results of prior laboratory tests
performed within an acceptable time window, and ordering new laboratory tests only if necessary) and calculation of eGFR. However,
if the patient is on dialysis or has known AKI, laboratory testing and calculation of eGFR is not useful or necessary (i.e., eGFR is not
accurate in this setting, and these patients would be considered at risk for NSF prior to group I or group III administration regardless
of calculated eGFR).
Calculating eGFR
For adults, eGFR calculation is commonly performed using the Modification of Diet in Renal Disease (MDRD) equation or the
Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation. The four- variable MDRD equation takes into account
age, gender, and serum creatinine level. The updated Schwartz equation should be used for children (also see Chapter on Contrast
Media in Children).
A number of websites and point-of-service tools are available that can calculate eGFR values in adults and children. For consistency,
radiologists may wish to identify which equation(s) are in use in their laboratory facilities.
When eGFR is recommended in outpatients with risk factor(s) for compromised renal function
For those patients identified by screening to have one or more risk factors for compromised renal function and in whom administration
of a group I or group III agent is planned, there is no high-level scientific evidence to guide the optimal time interval between eGFR
determination and GBCA injection. Nevertheless, the ACR Committee on Drugs and Contrast Media has made recommendations
(see Table 2) that take into consideration the need to maintain patient safety while minimizing the burden associated with excessive
laboratory testing.

ADVERSE REACTIONS TO GADOLINIUM-BASED CONTRAST MEDIA 78
Identifying at-risk inpatients
For all inpatients, an eGFR level should be obtained within 2 days prior to planned administration of a group I or group III GBCA.
In addition, ordering health professionals should assess inpatients for the possibility of AKI, as eGFR calculation alone has limited
accuracy for the detection of AKI.
General Recommendations for Imaging Patients at Risk for NSF
Group II agents are strongly preferred in patients at risk for NSF. Given the very low, if any, risk of NSF development with group II
agents, regardless of renal function or dialysis status, informed consent is not recommended prior to GBCA group II injection, but
deference is made to local practice preferences.
If use of a group I or group III agent is being considered in a patient with a risk of NSF, the potential benefit of a GBCA-enhanced
MRI exam are felt to outweigh the risk of NSF in an individual patient, and there is no suitable alternative, the referring physician
and patient should be informed of the risks of GBCA administration, and both should agree with the decision to proceed with GBCA
injection. Group I agents (see Table 1), the GBCAs that have been most often associated with NSF, have been contraindicated by the
FDA for use in these patients [26].
The lowest dose of GBCA required to obtain the needed clinical information should be used in at-risk patients, and it should generally
not exceed the recommended single dose. (Note: the lowest diagnostic dose has not been thoroughly investigated for many
indications; be careful not to minimize dose below diagnostic quality).
Exceptions to the above recommendations may be made at the discretion of the supervising radiologist, such as in the rare instance
of an acute, life-threatening condition, and after consultation with the referring health care professional. Documentation of the
rationale for the exception is recommended.
Limiting use of GBCAs in at-risk patients has already had a dramatic effect in reducing or even eliminating the number of new cases
of NSF [45]. It must be remembered that the risks of administering a GBCA to a high- risk patient must always be balanced against
the often substantial risks of not performing a needed contrast- enhanced imaging procedure.
Multiple doses of GBCA
In unusual circumstances, it may be necessary to administer multiple doses of a GBCA within a relatively short time frame. Examples
include a rapid change in patient condition for which an additional enhanced MR exam may be of benefit or when the initial MR
exam indicates an acute need for a more sophisticated enhanced MR exam. In patients not at risk of NSF, there is no contraindication
if the examination(s) are determined to be necessary. In patients at risk of NSF, the committee recommends the use of group II
agent(s).
Additional Specific Recommendations for Specific Groups of Patients
Patients with end-stage renal disease on chronic dialysis
If a contrast-enhanced cross-sectional imaging study is required in an anuric patient with no residual renal function, it would be
reasonable to consider administering iodinated contrast media and performing a CT rather than an MRI, assuming the anticipated
diagnostic yield is similar.
If a contrast-enhanced MR examination is to be performed in a patient with end-stage renal disease on chronic dialysis, injection of
group I agents (see Table 1) is contraindicated, and the committee recommends the use of a group II agent. When using a group II
agent, the risk of NSF is extremely low. The ACR Committee on Drugs and Contrast Media and National Kidney Foundation also
recommend that elective GBCA-enhanced MRI examinations be performed as closely before a regularly scheduled hemodialysis as
is possible, das dialysis can improve GBCM clearance. However, due to associated increased risk of catheter placement and infection,
the possibility of worsening kidney function in patients with AKI and CKD, the perceived very low risk of NSF from group II and
III GBCM agents, dialysis should not be initiated or altered (i.e. daily dialysis or multiple per-day dialysis sessions) [46,47].
Peritoneal dialysis may provide less NSF risk reduction compared to hemodialysis, but this has not been adequately studied.
Patients with CKD 4or5 (eGFR<30mL/min/1.73m2) not on chronic dialysis
Group I agents are contraindicated in this setting. If a GBCA-enhanced MRI study is to be performed, a group II agent should be
used.
ADVERSE REACTIONS TO GADOLINIUM-BASED CONTRAST MEDIA 79
Patients with CKD 3 (eGFR 30 to 59 mL / min/1.73 m2)
NSF developing after GBCA administration to patients with stable eGFR 30-59 ml/min/1.73 m2 is exceedingly rare. No special
precautions are necessary in this group [48,49].
Patients with CKD 1or2 (eGFR60to119mlmin/1.73m2)
There is no evidence that patients in these groups are at increased risk of developing NSF. Any GBCA can be administered safely to
these patients.
Patients with acute kidney injury (AKI)
Patients with AKI who have been exposed to GBCA are at risk for developing NSF [19]. Due to the temporal lag between eGFR
(which is calculated using serum creatinine values) and actual glomerular filtration rates, it is not possible to determine whether a
given patient has AKI based on a single eGFR determination. Accordingly, group I agents should be avoided in patients with known
or suspected AKI. If GBCA is to be administered in this setting, a group II agent is preferred.
Children
A systematic search of databases published in 2014 [50] found only 23 reported pediatric cases of NSF, and no cases in children
under the age of 6 years. Nevertheless, there is not enough data to demonstrate that NSF is less likely to occur in children than in
adults with similarly significant renal disease. Therefore, it is prudent to follow the same guidelines for adult and pediatric patients
as described in the remainder of this document. However, eGFR values in certain premature infants and neonates may be <30
ml/min/1.73 m2 simply due to immature renal function (and not due to pathologic renal impairment). In these individuals, the ACR
Committee on Drugs and Contrast Media believes that caution should still be used when administering GBCAs, and group II agents
should be used in this setting if feasible.
Caveat
Information on NSF and its relationship to GBCA administration continues to evolve, and the summary included here represents only
the most recent opinions of the ACR Committee on Drugs and Contrast Media. If additional information becomes available, our
understanding of causative events leading to NSF and recommendations for preventing it may change, leading to further revisions of
this document.
ADVERSE REACTIONS TO GADOLINIUM-BASED CONTRAST MEDIA 80
TABLE 1. ACR Manual Classification of Gadolinium-Based Agents Relative to Nephrogenic Systemic Fibrosis
Group I: Agents associated with the greatest number of NSF cases:
• Gadodiamide (Omniscan® – GE Healthcare)
• Gadopentetate dimeglumine (Magnevist® – Bayer HealthCare Pharmaceuticals)
• Gadoversetamide (OptiMARK® – Guerbet)
Group II: Agents associated with few, if any, unconfounded cases of NSF:
• Gadobenate dimeglumine (MultiHance® – Bracco Diagnostics)
• Gadobutrol (Gadavist® – Bayer HealthCare Pharmaceuticals; Gadovist in many countries)
• Gadoteric acid (Dotarem® – Guerbet, Clariscan – GE Healthcare)
• Gadoteridol (ProHance® – Bracco Diagnostics)
• Gadopiclenol* (Elucirem® – Guerbet, Vueway® – Bracco Diagnostics)
• Gadoxetate disodium (Eovist – Bayer HealthCare Pharmaceuticals; Primovist in many countries)
Group III: Agents for which data remains limited regarding NSF risk, but for which few, if any unconfounded cases of NSF have been
reported:
• No agents currently in this category (as of April 2024)
*
Gadopiclenol demonstrates kinetic stability and a long dissociation half-life that are comparable to other Group II macrocyclic agents. Based on the most recent scientific
and clinical evidence [47-50], the ACR Committee on Drugs and Contrast Media considers the risk of NSF among patients exposed to standard or lower than standard doses
of Gadopiclenol is sufficiently low or possibly nonexistent such that it has been classified as a Group II agent.
TABLE 2. eGFR Evaluation of Renal Function to Group I or Group III GBCA Administration
Patient Condition
eGFR Requirement
Patient on dialysis (any type)
No eGFR required — eGFR is not helpful in this situation.
Patient with AKI
No eGFR required — eGFR is not helpful in this situation.
Inpatient
Obtain eGFR within 2 days of the MRI study.
Outpatient/ED with no prior eGFR at the time the MR exam is
scheduled
If NO risk factors [1], no eGFR required.
WITH risk factors [1], obtain eGFR.*
Outpatient/ED with most recent prior eGFR of 45 or above
If NO risk factor [1] and eGFR of 60 or above, no new eGFR is required.
WITH risk factors [1] and/or eGFR 45-59, if most recent prior eGFR is within 6 weeks of
the MRI, no new eGFR is needed; otherwise obtain a new eGFR.**
Outpatient/ED with most recent prior eGFR of 44 or below
Obtain eGFR within 2 days of the MRI study
** If the new eGFR is to be obtained expressly for evaluation of suitability for administration of GBCA, obtaining the eGFR within 2 days of the MRI exam would avoid the
situation where the new eGFR might be less than 45 and require another eGFR within two days of the MRI exam, as per the last line in the table.
[1] Risk Factors:
1.
History of renal disease, including:
a.
Prior dialysis
b.
Renal transplant
c.
Single kidney
d.
Kidney surgery
e.
Renal cancer
2.
Diabetes mellitus
(optional)
References
1.
Grobner T. Gadolinium--a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol Dial
Transplant. 2006;21(4):1104-1108.
ADVERSE REACTIONS TO GADOLINIUM-BASED CONTRAST MEDIA 81
2.
Marckmann P, Skov L, Rossen K, et al. Nephrogenic systemic fibrosis: suspected causative role of gadodiamide used for contrast-enhanced
magnetic resonance imaging. J Am Soc Nephrol. 2006;17(9):2359-2362.
3.
Broome DR, Girguis MS, Baron PW, Cottrell AC, Kjellin I, Kirk GA. Gadodiamide -associated nephrogenic systemic fibrosis: why radiologists
should be concerned. AJR Am J Roentgenol 2007;188(2):586-592.
4.
Sadowski EA, Bennett LK, Chan MR, et al. Nephrogenic systemic fibrosis: risk factors and incidence estimation. Radiology. 2007;243(1):148-157.
5.
Shabana WM, Cohan RH, Ellis JH, et al. Nephrogenic systemic fibrosis: a report of 29 cases. AJR Am J Roentgenol
2008;190(3):736-741.
6.
Wertman R, Altun E, Martin DR, et al. Risk of nephrogenic systemic fibrosis: evaluation of gadolinium chelate contrast agents at four
American universities. Radiology 2008;248(3):799-806.
7.
European Medicines Agency. Questions and answers on the review of gadolinium-containing contrast agents. 2009; Available at:
http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/
gadolinium
_31/WC500015635.pdfAccessed4/18/2017.
8.
Gadolinium-Based Contrast Agents & Nephrogenic Systemic Fibrosis FDA Briefing Document. Joint Meeting of the Cardiovascular
and Renal Drugs and Drug Safety and Risk Management Advisory Committee. 2011; Available at
http://www.fda.gov/downloads/Advisory
Committees/CommitteesMeetingMaterials/Drugs/
DrugSafetyandRiskManagementAdvisoryCommittee/UCM190850.pdf. Accessed 4/18/2017.
9.
Collidge TA, Thomson PC, Mark PB, et al. Gadolinium-enhanced MR imaging and nephrogenic systemic fibrosis: retrospective study of a
renal replacement therapy cohort. Radiology 2007;245(1):168-175.
10.
Shibui K, KH, Sato N, Watanabe Y, Kohara M, Mochizuki T. A case of NSF attributable to contrast MRI repeated in a patient wit h Stage 3
CKD at a renal function of eGFR > 30 mL / min/1.73 m
2
. Japanese Journal of Nephrology 2009; 51:676.
11.
Abu-Alfa AK. Nephrogenic systemic fi and gadolinium-based contrast agents. Adv Chronic Kidney Dis 2011;18(3):188-198.
12.
Prince MR, Zhang H, Morris M, et al. Incidence of nephrogenic systemic fibrosis at two large medical centers. Radiology. 2008;248(3):807-816.
13.
Kalb RE, Helm TN, Sperry H, Thakral C, Abraham JL, Kanal E. Gadolinium-induced nephrogenic systemic fibrosis in a patient with an
acute and transient kidney injury. Br J Dermatol 2008;158(3):607-610.
14.
Pryor JG, Scott GA. Nephrogenic systemic fibrosis: a clinicopathologic study of 6 cases. JAm Acad Dermatol 2007;57(5):902- 903.
15.
Wahba IM, Simpson EL, White K. Gadolinium is not the only trigger for nephrogenic systemic fibrosis: insights from two cases and review of the
recent literature. Am J Transplant 2007;7(10):2425-2432.
16.
Weiss AS, Lucia MS, Teitelbaum I. A case of nephrogenic fibrosing dermopathy/nephrogenic systemic fibrosis. Nat Clin Pract Nephrol
2007;3(2):111-115.
17.
Kallen AJ, Jhung MA, Cheng S, et al. Gadolinium-containing magnetic resonance imaging contrast and nephrogenic systemic fibrosis: a
case-control study. Am J Kidney Dis 2008; 51:966-975.
18.
Bridges MD, St Amant BS, McNeil RB, Cernigliaro JG, Dwyer JP, Fitzpatrick PM. High -dose gadodiamide for catheter angiography and CT in
patients with varying degrees of renal insufficiency: Prevalence of subsequent nephrogenic systemic fibrosis and decline in renal function. AJR Am J
Roentgenol 2009; 192:1538-1543.
19.
Peak AS, Sheller A. Risk factors for developing gadolinium-induced nephrogenic systemic fibrosis. Ann Pharmacother
2007;41(9):1481-1485.
20.
High WA, Ayers RA, Chandler J, Zito G, Cowper SE. Gadolinium is detectable within the tissue of patients with nephrogenic systemic
fibrosis. J Am Acad Dermatol 2007;56(1):21-26.
21.
Swartz RD, Crofford LJ, Phan SH, Ike RW, Su LD. Nephrogenic fibrosing dermopathy: a novel cutaneous fibrosing disorder in pat ients with
renal failure. Am J Med 2003;114(7):563-572.
22.
Golding LP, Provenzale JM. Nephrogenic systemic fibrosis: possible association with a predisposing infection. AJR Am J Roentgenol 2008;
190:1069-1075.
23.
Wiginton CD, Kelly B, Oto A, et al. Gadolinium-based contrast exposure, nephrogenic systemic fibrosis, and gadolinium detection in tissue.
AJR Am J Roentgenol 2008;190(4):1060-1068.
24.
US Food and Drug Administration. Information for healthcare professionals: Gadolinium-based contrast agents for magnetic resonance imaging
(marketed as Magnevist, MultiHance, Omniscan, OptiMARK, ProHance). 2011; Available at:
http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm142884.htm
.
Accessed Sept. 20, 2011.
25.
Mazhar SM, Shiehmorteza M, Kohl CA, Allen J, Middleton MS, Sirlin CB. Nephrogenic systemic fibrosis in liver disease: a systematic review. J
Magn Reson Imaging. 2009;30(6):1313-1322.
26.
Abraham JL, Thakral C, Skov L, Rossen K, Marckmann P. Dermal inorganic gadolinium concentrations: evidence for in vivotransmetallation and
long-term persistence in nephrogenic systemic fibrosis. Br J Dermatol. 2008;158(2):273-280.
27.
Rosenkranz AR, Grobner T, Mayer GJ. Conventional or Gadolinium containing contrast media: the choice between acute renal failure or
Nephrogenic Systemic Fibrosis? Wien Klin Wochenschr. 2007;119(9-10):271-275.
28.
Christensen KN, Lee CU, Hanley MM, Leung N, Moyer TP, Pittelkow MR. Quantification of gadolinium in fresh skin and serum samples from
patients with nephrogenic systemic fibrosis. J Am Acad Dermatol. 2011;64(1):91-96.
29.
Rofsky NM, Sherry AD, Lenkinski RE. Nephrogenic systemic fi a chemical perspective. Radiology. 2008;247(3):608-612.
30.
Amet S, Launay-Vacher V, Clement O, et al. Incidence of nephrogenic systemic fibrosis in patients undergoing dialysis after contrast -enhanced
magnetic resonance imaging with gadolinium-based contrast agents: The Prospective Fibrose Nephrogenique Systemique study. Invest Radiol.
2014;49(2):109-115.
31.
Bruce R, Wentland AL, Haemel AK, et al. Incidence of Nephrogenic Systemic Fibrosis Using Gadobenate Dimeglumine in 1423 Patients With
Renal Insufficiency Compared With Gadodiamide. Invest Radiol. 2016;51(11):701-705.
32.
Martin DR, Krishnamoorthy SK, Kalb B, et al. Decreased incidence of NSF in patients on dialysis after changing gadolinium contrast-enhanced MRI
protocols. J Magn Reson Imaging. 2010;31(2):440-446.
33.
Michaely HJ, Aschauer M, Deutschmann H, et al. Gadobutrol in Renally Impaired Patients: Results of the GRIP Study. Invest Radiol.
2017;52(1):55-60.
34.
Nandwana SB, Moreno CC, Osipow MT, Sekhar A, Cox KL. Gadobenate Dimeglumine Administration and Nephrogenic Systemic Fibrosis: Is
ADVERSE REACTIONS TO GADOLINIUM-BASED CONTRAST MEDIA 82
There a Real Risk in Patients with Impaired Renal Function? Radiology. 2015;276(3):741-747.
35.
Reilly RF. Risk for nephrogenic systemic fibrosis with gadoteridol (ProHance) in patients who are on long-term hemodialysis. Clin J Am Soc Nephrol.
2008;3(3):747-751.
36.
Soulez G, Bloomgarden DC, Rofsky NM, et al. Prospective Cohort Study of Nephrogenic Systemic Fibrosis in Patients With Stage 3-5 Chronic
Kidney Disease Undergoing MRI With Injected Gadobenate Dimeglumine or Gadoteridol. AJR Am J Roentgenol. 2015;205(3):469-478.
37.
Wang Y, Alkasab TK, Narin O, et al. Incidence of nephrogenic systemic fibrosis after adoption of restrictive gadolinium - based contrast agent
guidelines. Radiology. 2011;260(1):105-111.
38.
Kanal E, Barkovich AJ, Bell C, et al. ACR guidance document for safe MR practices: 2007. AJR Am J Roentgenol. 2007;188(6):1447-1474.
39.
Kuo PH, Kanal E, Abu-Alfa AK, Cowper SE. Gadolinium-based MR contrast agents and nephrogenic systemic fibrosis. Radiology.
2007;242(3):647-649.
40.
Coresh J, Byrd-Holt D, Astor BC, et al. Chronic kidney disease awareness, prevalence, and trends among U.S. adults, 1999 to 2000. J Am Soc
Nephrol. 2005;16(1):180-188.
41.
Choyke PL, Cady J, DePollar SL, Austin H. Determination of serum creatinine prior to iodinated contrast media: is it necessary in all patients? Tech
Urol. 1998;4(2):65-69.
42.
Tippins RB, Torres WE, Baumgartner BR, Baumgarten DA. Are screening serum creatinine levels necessary prior to outpatient CT examinations?
Radiology. 2000;216(2):481-484.
43.
Altun E, Martin DR, Wertman R, Lugo-Somolinos A, Fuller ER, 3rd, Semelka RC. Nephrogenic systemic fibrosis: change in incidence following a
switch in gadolinium agents and adoption of a gadolinium policy--report from two U.S. universities. Radiology. 2009;253(3):689-696.
44.
Poggio ED, Nef PC, Wang X, et al. Performance of the Cockcroft-Gault and modification of diet in renal disease equations in estimating GFR in ill
hospitalized patients. Am J Kidney Dis. 2005;46(2):242-252.
45.
Skluzacek PA, Szewc RG, Nolan CR, 3rd, Riley DJ, Lee S, Pergola PE. Prediction of GFR in liver transplant candidates. Am J Kidney Dis.
2003;42(6):1169-1176.
46.
Nardone B, Saddleton E, Laumann AE, et al. Pediatric nephrogenic systemic fibrosis is rarely reported: a RADAR report. Pediatr Radiol.
2014;44(2):173-180.
47.
Robic C, Port M, Rousseaux O, Louguet S, Fretellier N, Catoen S, Factor C, Le Greneur S, Medina C, Bourrinet P, Raynal I, Idée JM, Corot C.
Physicochemical and Pharmacokinetic Profiles of Gadopiclenol: A New Macrocyclic Gadolinium Chelate With High T1 Relaxivity. Invest Radiol.
2019 Apr 4. doi:10.1097/RLI.0000000000000563. [Epub ahead of print]
48.
Fretellier N, Rasschaert M, Bocanegra J, Robert P, Factor C, Seron A, Idée JM, Corot C. Safety and Gadolinium Distribution of the New High-
Relaxivity Gadolinium Chelate Gadopiclenol in a Rat Model of Severe Renal Failure. Invest Radiol. 2021 Dec 1;56(12):826-836.
49.
Groden C, Pichiecchio A, Buki A. Efficacy and safety of gadopiclenol for central nervous system (CNS) magnetic resonance imaging (MRI): the
PICTURE trial. ECR (European Congress of Radiology); Vienna 2022 July 13-17 abstract RPS 2311-5
50.
Jurkiewicz E, Tsvetkova S, Grinberg A, Pasquiers B. Pharmacokinetics, Safety, and Efficacy of
Gadopiclenol in Pediatric Patients Aged 2 to 17 Years. Invest Radiol. 2022 Mar 21. doi:
10.1097/RLI.0000000000000865. Epub ahead of print. PMID: 35318970
Revision History
April 2023: Minor Revisions
July 2021: Updated to reflect the ACR-NKF Consensus Statement
15 May 2017: Major revisions
ULTRASOUND CONTRAST MEDIA 83
ULTRASOUND CONTRAST MEDIA
Contrast agents available for ultrasound, consisting of microbubbles or microspheres, allow for transient improvement in
ultrasound contrast resolution, increased conspicuity of vascularity, and detection of blood flow.
With dedicated ultrasound software, these contrast agents, composed of an outer phospholipid or protein wall and a central
inert echogenic gas, enhance the acoustic ultrasound signal from blood. Such contrast agents can be safely injected
intravenously through a peripheral or central line, or instilled into hollow structures, such as the urinary bladder.
Approved Agents and Uses
There are three ultrasound contrast agents with FDA approval available in the United States [1-3]:
1.
Definity
®
(perflutren lipid microspheres)
2.
Lumason
®
(sulfur hexafluoride lipid-type A microspheres; also known as SonoVue
®
)
3.
Optison
®
(perflutren protein-type A)
At the time of this publication, Definity, Lumason, and Optison are approved for intravenous administration in adults
undergoing echocardiography to improve visualization of the left ventricular cavity and its endocardial borders. Lumason is
also approved for liver imaging in both children and adults, and for imaging of the pediatric urinary tract for evaluation of
suspected or known vesicoureteral reflux (voiding ultrasonography) [4-7].
In addition to these approved indications, ultrasound contrast agents also have been used off-label to assess for the presence
and dynamics of blood flow in tumors [8], to differentiate benign cysts from solid masses in the kidney [9], to detect solid
organ injury in the setting of trauma [10-12], to detect and characterize endoleaks after abdominal aortic aneurysm repair
[13,14], to detect bowel wall inflammation in Crohn’s disease [15-18], to discriminate abscess from phlegmon [19], and to
guide and monitor ultrasound- guided interventions and ablative therapies [20]. Some of these off-label indications are in
clinical use, while others remain investigatory.
Contrast Agent Administration and Ultrasound Imaging
As detailed in the package labeling, these contrast agents are approved for intravenous slow infusion and/or bolus injection.
After reconstitution, they are typically hand injected through a moderate- or large- bore peripheral intravenous catheter
followed by a saline flush. The maximum volume of contrast material that can be administered per injection and per imaging
session differs by contrast agent.
Dedicated ultrasound software is available from most ultrasound vendors. Such software functions to suppress the background
tissue signal and maximize the signal from the contrast agent due to a combination of soundwave reflection and microbubble
resonance.
The likelihood of bubble rupture can and should be minimized by avoiding small intravenous catheters
and using a low mechanical index during imaging. The safety of high mechanical index imaging (>0.8) has not been well
studied and can cause microbubble cavitation or rupture.
Pharmacodynamics and Pharmacokinetics
Intravascularly administered ultrasound contrast agents generally remain in the blood pool because they are too large to enter
the interstitium (mean microbubble diameter: Definity [1.1-3.3 μm], Lumason [1.5-2.5 μm], Optison [3.0-4.5 μm). This
property differs from most CT and MRI contrast media.
Real-time assessment can be obtained over an approximately 10-minute period. After this time, the microbubbles
spontaneously rupture and dissolve, releasing an inert gas that is mostly eliminated through the lungs.
ULTRASOUND CONTRAST MEDIA 84
Safety Profile
Ultrasound contrast agents are safe, with an adverse event rate similar to or less than that of modern CT and MRI contrast
agents. A large retrospective investigation of more than 78,000 doses of Definity and Optison found a severe reaction rate
of 0.01% (n=8); half of these reactions (4 of 8) were considered anaphylactoid and there were no deaths [21]. Another large
retrospective study evaluating the use of SonoVue in 23,188 subjects documented 29 adverse events, with only two
considered serious [22]. The majority of adverse events are mild and likely physiologic in etiology, including symptoms
such as headache, a sensation of warmth or flushing, nausea, and altered taste. The majority of severe reactions occur within
30 minutes of administration.
A review performed by the Society for Pediatric Radiology in conjunction with the International Contrast Ultrasound Society
concluded that noncardiac applications of contrast-enhanced ultrasound—including intravenous and intravesical
administration—are safe, with side effects uncommon and typically minor [23]. A study [24] showed that that the intravenous
injection of microspheres is also well-tolerated in the pediatric population, with only minor side effects.
Ultrasound contrast agents are contraindicated for intra-arterial injection and in patients with previous hypersensitivity
reaction to microspheres. Old labeling included a contraindication for patients with a known or suspected right -to-left or
bidirectional shunt, but that labeling has been removed from the package inserts by the FDA, and it is no longer considered a
contraindication.
The risk for a serious cardiopulmonary reaction may be increased in patients who also have an unstable cardiopulmonary
condition (e.g., acute myocardial infarction, unstable congestive heart failure). However, a study [25] of 1513 hospitalized
patients with pulmonary hypertension showed that adverse reactions to Definity were very rare (0.002%).
Ultrasound contrast agents have no known renal toxicity in approved doses. There are no adequate and well- controlled studies
of these agents in pregnant women. While there are no known risks, these agents should only be used when needed and the
benefits outweigh any potential small risks to the fetus. As the effects of these contrast agents on human breast milk is
currently unknown, temporary (~24 hours) pumping and discarding of milk may be considered. Optison contains human
albumin, a derivative of human blood, and may confer a theoretical risk of viral or prion infection; additionally, it may be
contraindicated in patients with religious or ethical objections to the intravascular receipt of human blood products.
As with all contrast agents, appropriate resuscitation equipment and trained personnel should be readily available at the time
of injection in the event that an adverse reaction occurs.
References
1.
Optison™ (perflutren protein-type A). 2017; Available at: http://www3.gehealthcare.com/en/products/categories/ contrast media/optison.
Accessed April 10, 2017.
2.
Lumason® 2017; Available at: http://imaging.bracco.com/us-en/lumason. Accessed April 10, 2017.
3.
Definity® 2017; Available at: http://www.definityimaging.com/main.html. Accessed April 10, 2017.
4.
Darge K, Troeger J. Vesicoureteral reflux grading in contrast-enhanced voiding urosonography. Eur J Radiol. 2002;43(2):122-128.
5.
Fernandez-Ibieta M, Parrondo-Muinos C, Fernandez-Masaguer LC, et al. Voiding urosonography with second-generation contrast as a main tool
for examining the upper and lower urinary tract in children. Pilot Study. Actas Urol Esp. 2016;40(3):183-189.
6.
Papadopoulou F, Anthopoulou A, Siomou E, Efremidis S, Tsamboulas C, Darge K. Harmonic voiding urosonography with a second -generation
contrast agent for the diagnosis of vesicoureteral reflux. Pediatr Radiol. 2009;39(3):239-244.
7.
Radmayr C, Klauser A, Pallwein L, Zurnedden D, Bartsch G, Frauscher F. Contrast enhanced reflux sonography in children: a com parison to
standard radiological imaging. J Urol. 2002;167(3):1428-1430.
8.
McCarville MB, Coleman JL, Guo J, et al. Use of Quantitative Dynamic Contrast-Enhanced Ultrasound to Assess Response to Antiangiogenic
Therapy in Children and Adolescents with Solid Malignancies: A Pilot Study. AJR Am J Roentgenol. 2016;206(5):933-939.
9.
Barr RG, Peterson C, Hindi A. Evaluation of indeterminate renal masses with contrast-enhanced US: a diagnostic performance study.
Radiology. 2014;271(1):133-142.
10.
Durkin N, Deganello A, Sellars ME, Sidhu PS, Davenport M, Makin E. Post-traumatic liver and splenic pseudoaneurysms in children:
Diagnosis, management, and follow-up screening using contrast enhanced ultrasound (CEUS). J Pediatr Surg. 2016;51(2):289-292.
11.
Miele V, Piccolo CL, Galluzzo M, Ianniello S, Sessa B, Trinci M. Contrast-enhanced ultrasound (CEUS) in blunt abdominal trauma. Br J
Radiol. 2016;89(1061):20150823.
12.
Mihalik JE, Smith RS, Toevs CC, Putnam AT, Foster JE. The use of contrast-enhanced ultrasound for the evaluation of solid abdominal organ
injury in patients with blunt abdominal trauma. J Trauma Acute Care Surg. 2012;73(5):1100-1105.
13.
Carrafiello G, Recaldini C, Lagana D, Piffaretti G, Fugazzola C. Endoleak detection and classification after endovascular treatment of abdominal
aortic aneurysm: value of CEUS over CTA. Abdom Imaging. 2008;33(3):357-362.
14.
Karthikesalingam A, Al-Jundi W, Jackson D, et al. Systematic review and meta-analysis of duplex ultrasonography, contrast-enhanced
ultrasonography or computed tomography for surveillance after endovascular aneurysm repair. Br J Surg. 2012;99(11):1514-1523.
15.
De Franco A, Di Veronica A, Armuzzi A, et al. Ileal Crohn disease: mural microvascularity quantified with contrast-enhanced US correlates
with disease activity. Radiology. 2012;262(2):680-688.
ULTRASOUND CONTRAST MEDIA 85
16.
Horjus Talabur Horje CS, Bruijnen R, Roovers L, Groenen MJ, Joosten FB, Wahab PJ. Contrast Enhanced Abdominal Ultrasound in the
Assessment of Ileal Inflammation in Crohn’s Disease: A Comparison with MR Enterography. PLoS One. 2015;10(8): e0136105.
17.
Quaia E, Cabibbo B, De Paoli L, Toscano W, Poillucci G, Cova MA. The value of time-intensity curves obtained after microbubble contrast
agent injection to discriminate responders from non-responders to anti-inflammatory medication among patients with Crohn’s disease. Eur
Radiol. 2013;23(6):1650-1659.
18.
Quaia E, Migaleddu V, Baratella E, et al. The diagnostic value of small bowel wall vascularity after sulfur hexafluoride - filled microbubble
injection in patients with Crohn’s disease. Correlation with the therapeutic effectiveness of specific anti- inflammatory treatment. Eur J Radiol.
2009;69(3):438-444.
19.
Ripolles T, Martinez-Perez MJ, Paredes JM, Vizuete J, Garcia-Martinez E, Jimenez-Restrepo DH. Contrast-enhanced ultrasound in the
differentiation between phlegmon and abscess in Crohn’s disease and other abdominal conditions. Eur J Radiol. 2013;82(10): e525-531.
20.
Meloni MF, Smolock A, Cantisani V, et al. Contrast enhanced ultrasound in the evaluation and percutaneous treatment of hepatic and renal
tumors. Eur J Radiol. 2015;84(9):1666-1674.
21.
Wei K, Mulvagh SL, Carson L, et al. The safety of deFinity and Optison for ultrasound image enhancement: a retrospective analysis of 78,383
administered contrast doses. J Am Soc Echocardiogr. 2008;21(11):1202-1206.
22.
Piscaglia F, Bolondi L, Italian Society for Ultrasound in M, Biology Study Group on Ultrasound Contrast A. The safety of Sono vue in
abdominal applications: retrospective analysis of 23188 investigations. Ultrasound Med Biol. 2006;32(9):1369-1375.
23.
Darge K, Papadopoulou F, Ntoulia A, et al. Safety of contrast-enhanced ultrasound in children for non-cardiac applications: a review by the
Society for Pediatric Radiology (SPR) and the International Contrast Ultrasound Society (ICUS). Pediatr Radiol. 2013;43(9):1063-1073.
24.
Coleman JL, Navid F, Furman WL, McCarville MB. Safety of ultrasound contrast agents in the pediatric oncologic population: a single-
institution experience. AJR Am J Roentgenol 2014;202:966-70.
25.
Wever-Pinzon O, Suma V, Ahuja A, et al. Safety of echocardiographic contrast in hospitalized patients with pulmonary hypertension: a multi-
center study. Eur Heart J Cardiovasc Imaging 2012;13:857-62.
TREATMENT OF CONTRAST REACTIONS 86
TREATMENT OF CONTRAST REACTIONS
Optimal treatment of contrast reactions starts with a well-designed plan and a properly trained staff. In addition to basic life
support training, on-site personnel should be trained in the rapid recognition, assessment, diagnosis, and treatment of contrast
reactions.
In evaluating a patient for a potential contrast reaction, five immediate assessments should be made if clinically feasible:
•
What is the patient’s general appearance?
•
Can the patient speak? How does their voice sound?
•
What is the quality of the patient’s breathing?
•
What is the patient’s pulse?
•
What is the patient’s blood pressure?
The patient’s level of consciousness, appearance of their skin, quality of phonation, lung auscultation, blood pressure, and heart
rate assessment will allow the responding provider to quickly determine the severity of a reaction and properly diagnose it.
Once diagnosed, effective treatment can be rapidly and effectively administered (see Tables 1, 4, and 3). Staff should be aware
of how to activate the emergency response system to elevate the level of care if needed—for example, calling an emergency
response phone number (e.g., 911 for emergency medical personnel assistance in an outpatient medical center setting).
Mild immediate reactions (both allergic-like and physiologic) typically do not require medical treatment. However, a mild
reaction may evolve into a moderate or severe reaction. Vital signs should be obtained to detect hypotension that may be
clinically silent while the patient is supine. Any patient with a mild allergic-like reaction should be observed for a minimum
of 20 to 30 minutes to ensure clinical stability or recovery. Treatment with an antihistamine may be instituted for mild
symptomatic allergic-like urticarial reactions, but often is not necessary.
Most moderate and all severe reactions will require prompt and aggressive treatment to reduce the likelihood of an adverse
outcome. Treatment algorithms for adults and children are provided in Tables 1, 2, and 3.
Ongoing quality assurance and quality improvement programs with in-service training and review sessions are helpful in
ensuring that responses to contrast reactions are prompt and appropriate. This includes training of onsite health care providers
in resuscitation techniques such as basic life support.
References
1.
Barach EM, Nowak RM, Lee TG, Tomlanovich MC. Epinephrine for treatment of anaphylactic shock. JAMA 1984; 251:2118- 2122.
2.
Bennett MJ, Hirshman CA. Epinephrine for anaphylactic shock. JAMA 1985; 253:510-511.
3.
Berg RA, et al. Part 5: adult basic life support: 2010 American Heart Association guidelines for cardio-pulmonary resuscitation and
emergency cardiovascular care. Circulation 2010; 22:15): S685-S705.
4.
Braddom RL, Rocco JF. Autonomic dysreflexia. A survey of current treatment. Am J Phys Med Rehabil 1991; 70:234-241.
5.
Brown JH. Atropine, scopolamine, and antimuscarinic drugs. In: Gilman AG, Rall TW, Nies AS, et al, ed. The pharmaceutical bas is of
therapeutics. New York, NY: Pergamon; 1990:150-165.
6.
Bush WH, Swanson DP. Acute reactions to intravascular contrast media: types, risk factors, recognition, and specific treatmen t. AJR
1991; 157:1153-1161.
7.
Bush WH. Treatment of acute contrast reactions. In: Bush WH, King B, Krecke K, ed. Radiology Life Support (RAD-LS). London:
Hodder Arnold Publishers; 1999.
8.
Chamberlain DA, Turner P, Sneddon JM. Effects of atropine on heart-rate in healthy man. Lancet 1967; 2:12-15.
9.
Cohan RH, Leder RA, Ellis JH. Treatment of adverse reactions to radiographic contrast media in adults. Radiol Clin North Am
1996; 34:1055-1076.
10.
Collins MS, Hunt CH, Hartman RP. Use of IV epinephrine for treatment of patients with contrast reactions: lessons learned from a 5-year
experience. AJR 2009; 192:455-461.
11.
Field JM, Hazinski MF, Sayre MR, et al. Part 1: executive summary: 2010 American Heart Association guidelines for
cardiopulmonary and emergency cardiovascular care. Circulation 2010;122: S640-S656.
12.
Grauer K, Cavallaro D. ACLS: certification preparation and a comprehensive review. Vol I and II. St. Louis, Mo: Mosby; 1993.
13.
Guidelines for cardiopulmonary resuscitation and emergency cardiac care. Emergency Cardiac Care Committee and Subcommittees,
American Heart Association. Part III. Adult advanced cardiac life support. JAMA 1992; 268:2199-2241.
14.
Hoffmann BB, Lefkowitz RJ. Catecholamines and sympathomimetic drugs. In: Gilman AG, Rall TW, Nies AS, et al, ed. The
pharmacological basis of therapeutics. New York, NY: Pergamon; 1990:192-198.
15.
McClennan BL. Adverse reactions to iodinated contrast media. Recognition and response. Invest Radiol 1994; 29 Suppl 1: S46- 50.
16.
Neumar RW, Otto CW, Link MS, et al. Part 8: adult advanced cardiovascular life support: 2010 American Heart Association
guidelines for cardio-pulmonary resuscitation and emergency cardiovascular care. Circulation 2010;122: S729-S767.
17.
Runge JW, Martinez JC, Caravati EM, Williamson SG, Hartsell SC. Histamine antagonists in the treatment of acute allergic reactions.
TREATMENT OF CONTRAST REACTIONS 87
Ann Emerg Med 1992; 21:237-242.
18.
Segal AJ, Bush WH, Jr. Avoidable errors in dealing with anaphylactoid reactions to iodinated contrast media. Invest Radiol
2011; 46:147-151.
19.
Swanson DP, Chilton HM, Thrall JH, ed. Pharmaceuticals in medical imaging. New York, NY: Macmillan; 1990.
20.
Travers AH, Rea TD, Bobrow BJ, et al. Part 4: CPR overview: 2010American Heart Association guidelines for cardiopulmonary
resuscitation and emergency cardiovascular care. Circulation 2010;122: S676-S684.
21.
vanSonnenberg E, Neff CC, Pfister RC. Life-threatening hypotensive reactions to contrast media administration: comparison of
pharmacologic and fluid therapy. Radiology 1987; 162:15-19.
Revision History
15 January 2020: Minor revisions
ADMINISTRATION OF CONTRAST MEDIA TO PREGNANT OR POTENTIALLY PREGNANT PATIENTS 88
ADMINISTRATION OF CONTRAST MEDIA TO PREGNANT OR
POTENTIALLY PREGNANT PATIENTS
Studies of low-molecular weight water-soluble extracellular substances such as iodinated and gadolinium- based contrast media
in pregnancy have been limited, and their effects on the human embryo or fetus are incompletely understood. Iodinated contrast
media have been shown to cross the human placenta and enter the fetus in measurable quantities [1,2]. A standard gadolinium-
based contrast medium has been shown to cross the placenta in primates and appear within the fetal bladder within 11 minutes
after intravenous (IV) administration [3]. It is likely that all iodinated and gadolinium-based contrast media behave in a similar
fashion and cross the blood-placental barrier and into the fetus.
After entering the fetal blood stream, these agents will be excreted via the urine into the amniotic fluid and be subsequently
swallowed by the fetus [4]. It is then possible that a small amount will be absorbed from the gut of the fetus, with the additional
swallowed gadolinium-based contrast agents eliminated back into the amniotic fluid.
In a study in primates, placental enhancement could be detected up to 2 hours following IV administration of gadopentetate
dimeglumine. When gadopentetate dimeglumine was injected directly into the amniotic cavity, it was still conspicuous at 1
hour after administration [3]. There are no data available to assess the rate of clearance of contrast media from the amniotic
fluid.
Iodinated Low-Osmolality Contrast Media
Mutagenic effect of low-osmolality contrast media
Diagnostic iodinated contrast media have been shown to cross the human placenta and enter the fetus when given in usual
clinical doses. In-vivo tests in animals have shown no evidence of either mutagenic or teratogenic effects with low-osmolality
contrast media (LOCM). No well-controlled studies of the teratogenic effects of these media in pregnant women have been
performed.
Effect of iodinated contrast media on fetal thyroid function
The fetal thyroid plays an important role in the development of the central nervous system. There have been rare reports of
hypothyroidism developing in the newborn infant after the administration of an iodinated contrast medium during pregnancy;
however, this occurred only following amniofetography using a fat- soluble iodinated contrast medium, which was performed
in the past to detect congenital malformations.
Intravenous administration of iodinated contrast media does not affect short-term neonatal thyroid stimulating hormone (TSH),
likely because the overall amount of excess iodide in the fetal circulation is small and transient. However, the long-term effects
are unknown. To date, there has been no documented case of neonatal hypothyroidism from the maternal intravascular injection
of water-soluble iodinated contrast agents [5,6]. Given the current available data and routine evaluation of all newborns for
congenital hypothyroidism by measurement of TSH levels at the time of their birth, no extra attention is felt to be necessary
[7-9].
Other adverse effects
No other adverse effects have been reported in the fetus or neonate following administration of LOCM. However, information
in this area is sparse.
Recommendations prior to performing imaging studies requiring iodinated contrast material administration
Given that there are no available data to suggest any potential harm to the fetus from exposure to iodinated contrast medium
via maternal IV or intra-arterial injection, we do not recommend routine screening for pregnancy prior to contrast media use.
This recommendation is also supported by the FDA classifying of most iodinated contrast agents as category B medications.
Screening for potential pregnancy in women of child-bearing age receiving radiation to the pelvis, which is discussed
separately, is therefore not affected by the use of iodinated contrast agents (See the ACR–SPR Practice Parameter for Imaging
Pregnant or Potentially Pregnant Adolescents and Women With Ionizing Radiation). We do not recommend withholding the
use of iodinated contrast agents in pregnant or potentially pregnant patients when it is needed for diagnostic purposes.
ADMINISTRATION OF CONTRAST MEDIA TO PREGNANT OR POTENTIALLY PREGNANT PATIENTS 89
Gadolinium-Based Contrast Agents
Mutagenic effect of gadolinium-based contrast agents
To date, there have been no known adverse effects to human fetuses reported when clinically recommended dosages of
gadolinium-based contrast agents (GBCAs) have been given to pregnant women. A single cohort study of 26 women exposed
to gadolinium chelates during the fi trimester of pregnancy showed no evidence of teratogenesis or mutagenesis in their progeny
[10]. However, no well-controlled studies of the teratogenic effects of these media in pregnant women have been performed.
In a retrospective review [11] of a Canadian provincial database of births, the risk of a congenital anomaly did not differ
between patients exposed to GBCAs at any time during pregnancy and those patients who did not undergo MRI.
Risk of nephrogenic systemic fibrosis
There are no known cases of nephrogenic systemic fibrosis (NSF) linked to the use of GBCAs in pregnant patients. However,
gadolinium chelates may accumulate in the amniotic fluid Therefore, there is the potential for the dissociation of the toxic free
gadolinium ion, conferring a potential risk for the development of NSF in the child or mother.
In a retrospective review [11] of a Canadian provincial database of births, exposure to GBCAs at any time during pregnancy
was associated with an increased risk in the child of a broad set of rheumatological, inflammatory, or infiltrative conditions.
With further analysis, only first-trimester GBCA exposure showed this association. However, the study had some substantial
limitations. The control group was patients who did not undergo MRI during pregnancy, rather than patients who underwent
MRI without GBCA. Also, the percentage of patients experiencing the condition was 31% in the GBCA exposed group and
27% in the unexposed group (adjusted hazard ratio 1.36), which was statistically significantly different given the large numbers
in the unexposed group, but is a surprisingly large baseline percentage in the unexposed group. The number of cases of a
connective tissue or skin disease resembling NSF was too small for statistical analysis. Whether any of the children were
exposed after birth to GBCA was not investigated.
Risk of still birth or neonatal death
In a retrospective review [11] of a Canadian provincial database of births, exposure to GBCAs at any time during pregnancy
was associated with an increased risk of stillbirth or neonatal death, although the number of deaths in the exposed group was
small. In addition, the control group was patients who did not undergo MRI during pregnancy, rather than patients who
underwent MRI without GBCA.
Recommendations for the use of GBCA-enhanced MRI examinations in pregnant patients
Because it is unclear how GBCAs will affect the fetus, these agents should be administered with caution to pregnant or
potentially pregnant patients. GBCAs should only be used if their usage is considered critical and the potential benefits justify
the potential unknown risk to the fetus. If a GBCA is to be used in a pregnant patient, one of the agents believed to be at low
risk for the development of NSF [12] should be used at the lowest possible dose to achieve diagnostic results. In pregnant
patients with severely impaired renal function, the same precautions should be observed as in non-pregnant patients. The ACR
Committee on Drugs and Contrast Media recommends the following concerning the performance of contrast- enhanced MRI
examinations in pregnant patients:
Each case should be reviewed carefully by members of the clinical and radiology service groups, and a GBCA should be
administered only when there is a potential significant benefit to the patient or fetus that outweighs the possible but unknown
risk of fetal exposure to free gadolinium ions.
A.
The radiologist should confer with the referring physician and document the following in the radiology report or the
patient’s medical record:
1.
That information requested from the MRI study cannot be acquired without the use of IV contrast or by using other
imaging modalities.
2.
That the information needed affects the care of the patient and/or fetus during the pregnancy.
3.
That the referring physician is of the opinion that it is not prudent to wait to obtain this information until after the
patient is no longer pregnant.
B.
It is recommended that informed consent be obtained from the patient after discussion with the referring physician.
ADMINISTRATION OF CONTRAST MEDIA TO PREGNANT OR POTENTIALLY PREGNANT PATIENTS 90
Premedication of pregnant patients (with prior allergic-like reactions to iodinated or gadolinium-based contrast
media)
Diphenhydramine and corticosteroids (most commonly prednisone and methylprednisolone) are commonly used for prophylaxis
in patients at risk for allergic-like contrast reactions to contrast media. Diphenhydramine is classified as FDA category B. (FDA
category B: Animal reproductive studies have failed to demonstrate a risk to the fetus, and there are no adequate well-controlled
studies in pregnant women.) Prednisone (FDA category C) and dexamethasone (FDA category C) traverse the placenta;
however, most of these agents are metabolized within the placenta before reaching the fetus and therefore are not associated
with teratogenicity in humans. (FDA category C: Animal reproductive studies have shown an adverse effect on fetus, and there
are no adequate and well-controlled studies in humans, but potential benefits may warrant use of the drug in pregnant women
despite potential risks.) However, sporadic cases of fetal adrenal suppression have been reported. Methylprednisolone is also
classified as a category C drug and carries a small risk to the fetus for the development of a cleft lip if used before 10 weeks of
gestation [13,14].
Recommendations for the use of corticosteroid premedication in pregnant patients
Expert opinion indicates that the use of steroids in pregnancy is generally safe [15,16], although common specific regimens for
premedication prior to contrast media administration have not been tested. Severe anaphylaxis in a pregnant female represents
an even greater risk to the fetus than to the mother herself [17]. Given this information, we recommend that otherwise-indicated
premedication to reduce the risk of contrast media reaction not be withheld because the patient is pregnant and a standard PO or
IV regimen be employed (see Chapter on Patient Selection and Preparation Strategies). Both referring clinicians and their
pregnant patients receiving premedication prior to contrast media administration should indicate that they understand the
potential risks and benefits of the medications being used, as well as alternative diagnostic options [18].
Management of contrast reactions in pregnant patients
The management of contrast reactions in pregnant patients is generally the same as that of contrast reactions in non- pregnant
adults [19], with minor additions.
For the treatment of hypotension in patients with an obviously gravid uterus, the patient may be placed in the left lateral decubitus
position or positioned supine with a leftward tilt using a wedge. If cardiac compressions are required, these are usually best
performed in the supine position; in this situation, manual displacement of the uterus upward and to the left is recommended (if
there are sufficient personnel to perform this maneuver). These tactics reduce the compression of the inferior vena cava by the
gravid uterus which may otherwise compromise venous return to the heart [19].
References:
1.
Dean PB. Fetal uptake of an intravascular radiologic contrast medium. Rofo. 1977;127(3):267-270.
2.
Moon AJ, Katzberg RW, Sherman MP. Transplacental passage of iohexol. J Pediatr. 2000;136(4):548-549.
3.
Panigel M, Wolf G, Zeleznick A. Magnetic resonance imaging of the placenta in rhesus monkeys, Macaca mulatta. J Med Primatol.
1988;17(1):3-18.
4.
Kanal E, Barkovich AJ, Bell C, et al. ACR guidance document for safe MR practices: 2007. AJR Am J Roentgenol. 2007;188(6):1447-
1474.
5.
Atwell TD, Lteif AN, Brown DL, McCann M, Townsend JE, Leroy AJ. Neonatal thyroid function after administration of IV iodinated
contrast agent to 21 pregnant patients. AJR Am J Roentgenol. 2008;191(1):268-271.
6.
Bourjeily G, Chalhoub M, Phornphutkul C, Alleyne TC, Woodfield CA, Chen KK. Neonatal thyroid function: effect of a single exposure to
iodinated contrast medium in utero. Radiology. 2010;256(3):744-750.
7.
Kochi MH, Kaloudis EV, Ahmed W, Moore WH. Effect of in utero exposure of iodinated intravenous contrast on neonatal thyroid
function. J Comput Assist Tomogr. 2012;36(2):165-169.
8.
McKay DB, Josephson MA. Pregnancy in recipients of solid organs--effects on mother and child. N Engl J Med. 2006;354(12):1281-
1293.
9.
Rajaram S, Exley CE, Fairlie F, Matthews S. Effect of antenatal iodinated contrast agent on neonatal thyroid function. Br J Radiol.
2012;85(1015): e238-242.
10.
De Santis M, Straface G, Cavaliere AF, Carducci B, Caruso A. Gadolinium periconceptional exposure: pregnancy and neonatal
outcome. Acta Obstet Gynecol Scand. 2007;86(1):99-101.
11.
Ray JG, Vermeulen MJ, Bharatha A, Montanera WJ, Park AL. Association between MRI exposure during pregnancy and fetal and
childhood outcomes. JAMA. 2016;316(9):952-961
12.
Thomsen HS, Morcos SK, Almen T, et al. Nephrogenic systemic fibrosis and gadolinium-based contrast media: updated ESUR
Contrast Medium Safety Committee guidelines. Eur Radiol. 2013;23(2):307-318.
13.
Niebyl JR. Clinical practice. Nausea and vomiting in pregnancy. N Engl J Med. 2010;363(16):1544-1550.
14.
Wang PI, Chong ST, Kielar AZ, et al. Imaging of pregnant and lactating patients: part 1, evidence-based review and recommendations.
AJR Am J Roentgenol. 2012;198(4):778-784.
15.
Bonanno C, Wapner RJ. Antenatal corticosteroids in the management of preterm birth: are we back where we started? Obstet Gynecol
ADMINISTRATION OF CONTRAST MEDIA TO PREGNANT OR POTENTIALLY PREGNANT PATIENTS 91
Clin North Am. 2012;39(1):47-63.
16.
Gerosa M, Meroni PL, Cimaz R. Safety considerations when prescribing immunosuppression medication to pregnant women.
Expert Opin Drug Saf. 2014;13(12):1591-1599.
17.
Simons FE, Schatz M. Anaphylaxis during pregnancy. J Allergy Clin Immunol. 2012;130(3):597-606.
18.
Horowitz JM, Bisla JK, Yaghmai V. Premedication of pregnant patients with history of iodinated contrast allergy. Abdom Radiol (NY).
2016;41(12):2424-2428.
19.
Sikka A, Bisla JK, Rajan PV, et al. How to manage allergic reactions to contrast agent in pregnant patients. AJR Am J Roentgenol.
2016;206(2):247-252.
Revision History
15 May 2017: Minor revisions
28 June 2015: Major revisions
23 June 2010: Minor revisions
ADMINISTRATION OF CONTRAST MEDIA TO WOMEN WHO ARE BREAST-FEEDING 92
Breast Feeding Recommendations with Associated Strength of Evidence
Below is a summary of the recommendations related to breast feeding and the associated strength of
evidence for those recommendations using the ACR Appropriateness Criteria® Methodology.
Question 1. Should breast feeding be routinely ceased for 24 hours after maternal IV or IA exposure to iodinated
contrast material?
Recommendation: Because of the very small percentage of iodinated contrast medium that is excreted into the breast milk
and absorbed by the infant’s gut (under 0.01%), the available data suggest that it is safe for the mother and infant to
continue breast-feeding after receiving such an agent. Therefore, stopping breastfeeding for 24 hours after maternal IV or
IA exposure to iodinated contrast material is not required. It is recommended patients make an informed decision on
managing breastmilk after exposure to iodinated contrast material.
Strength of Evidence: Limited
Reference
Name
Study Quality
[1]
Webb 2005
4
[2]
Trembley 2012
4
[3]
Wang 2012
4
[4]
Nielsen 1987
4
Question 2. Should breast feeding be routinely ceased for 24 hours after maternal IV or IA exposure to gadolinium-
based contrast agents?
Recommendation: Because of the very small percentage of gadolinium-based contrast medium that is excreted into the
breast milk and absorbed by the infant’s gut (estimated to be under 0.0004%), the available data suggest that it is safe for
the mother and infant to continue breast-feeding after receiving such an agent [5]. Therefore, stopping breastfeeding for 24
hours after maternal IV or IA exposure to iodinated contrast material is not required. It is recommended patients make an
informed decision on managing breastmilk after exposure to iodinated contrast material.
Strength of Evidence: Limited
Reference
Name
Study Quality
[6]
Rofsky 1993
4
[5]
Kubik-Huch 2000
3
[7]
Lin 2007
4
Question 3. Should routine thyroid function testing be performed in infants exposed to iodinated contrast material
through maternal breast milk?
Recommendation: No strong data exists in the literature that the amount of iodinated contrast material absorbed systemically
in an infant from maternal breast milk following a conventional contrast-enhanced CT is enough to place the infant at risk
for hypothyroidism. Therefore, routine thyroid function testing is not recommended in this clinical setting.
Strength of Evidence: Limited
Reference
Name
Study Quality
[8]
Themelin 2019
4

ADMINISTRATION OF CONTRAST MEDIA TO WOMEN WHO ARE BREAST-FEEDING 93
ADMINISTRATION OF CONTRAST MEDIA TO PATIENTS WHO ARE
BREAST-FEEDING
Imaging studies requiring either iodinated or gadolinium-based contrast media are occasionally required in patients who are
breast feeding. Both the patient and the patient’s physician may have concerns regarding potential toxicity to the infant from
contrast media that is excreted into the breast milk.
The literature on the excretion into breast milk of iodinated and gadolinium-based contrast media and the gastrointestinal
absorption of these agents from breast milk is very limited; however, several studies have shown that the expected dose of
contrast medium absorbed by an infant from ingested breast milk is extremely low.
Iodinated X-ray Contrast Media (Ionic and Nonionic)
Background
The plasma half-life of intravenously administered iodinated contrast medium is approximately 2 hours, with nearly 100% of
the media cleared from the bloodstream in patients with normal renal function within 24 hours. Because of its low lipid
solubility, less than 1% of the administered maternal dose of iodinated contrast medium is excreted into the breast milk in the
first 24 hours with one paper showing concentrations of iohexol (350 mg I/ml) of approximately 0.5% of weight-adjusted
maternal dose in the first 24 hours after exposure [1,4,9]. In addition, less than 1% of the contrast medium ingested by the infant
is absorbed from its gastrointestinal tract [2]. Therefore, the expected systemic dose absorbed by the infant from the breast milk
is less than 0.01% of the intravascular dose given to the mother. This amount represents less than 1% of the recommended dose
for an infant being prescribed iodinated contrast material intravenously related to an imaging study (usually 1.5 to 2 mL/kg).
The potential risks to the infant from breast milk include allergic sensitization or reaction, which are theoretical concerns but
have not been reported, and adverse effects on the infant’s thyroid. The likelihood of either direct toxic or allergic-like
manifestations resulting from ingested iodinated contrast material in the infant is extremely low. There is no high-quality
literature on thyroid dysfunction in infants related to contrast media exposure through breast milk with a single case report
inferring transient neonatal hypothyroidism induced by breast milk containing iodinated contrast media in a premature infant
(despite withholding breast milk for 24 hours post exposure) [8]. As with other medications in milk, the taste o f the milk may
be altered if it contains contrast medium [1,2,4,9].
For further information on ACR recommendations / guidelines on thyroid testing after direct iodinated contrast exposure in
infants please see the ACR Statement on Use of Iodinated Contrast Material for Medical Imaging in Young Children and Need
for Thyroid Monitoring and the Contrast Media in Children Chapter.
Recommendation
Because of the very small percentage of iodinated contrast medium that is excreted into the breast milk and absorbed by the
infant’s gut, the available data suggest that it is safe for the mother and infant to continue breast-feeding after receiving such an
agent. Ultimately, however, an informed decision to temporarily stop breast-feeding should be left up to the mother after these
facts are communicated. If the mother remains concerned about any potential ill effects to the infant, she may abstain from
breast-feeding from the time of contrast administration for a period of 12 to 24 hours. In this situation, the mother should be
told to express and discard breast milk from both breasts during that period. In anticipation of this, she may wish to use a breast
pump to obtain milk before the contrast-enhanced study to feed the infant during the 24-hour period following the examination.
There is little value to stop breast-feeding beyond 24 hours.
While data remains limited, we do not recommend routine thyroid monitoring in infants (premature or term) after exposure to
iodinated contrast material through ingested breast milk.
Gadolinium-Based Contrast Agents
Background
Like iodinated contrast media, gadolinium-based contrast media have a plasma half-life of approximately 2 hours and are nearly
completely cleared from the bloodstream in patients with normal renal function within 24 hours. Also similar to iodinated
contrast media, gadolinium-based contrast media are excreted into the breast milk. It is likely that the overwhelming bulk of
gadolinium excreted in the breast milk is in a stable and chelated form [5].
ADMINISTRATION OF CONTRAST MEDIA TO WOMEN WHO ARE BREAST-FEEDING 94
Less than 0.04% of the intravascular dose given to the mother is excreted into the breast milk in the first 24 hours [3,5,6].
Because less than 1% of the contrast medium ingested by the infant is absorbed from its gastrointestinal tract [5,7], the expected
systemic dose absorbed by the infant from the breast milk is less than 0.0004% of the intravascular dose given to the mother.
This ingested amount is far less than the permissible dose for intravenous use in neonates. The likelihood of an adverse effect
from such a minute fraction of gadolinium chelate absorbed from breast milk is remote [1]). However, the potential risks to the
infant include direct toxicity (including toxicity from free gadolinium, because it is unknown how much, if any, of the
gadolinium in breast milk is in the unchelated form) and allergic sensitization or reaction. These are theoretical concerns but
none of these complications have been reported [6]. As in the case with iodinated contrast medium, the taste of the milk may
be altered if it contains a gadolinium-based contrast medium [1].
Recommendation
Because of the very small percentage of gadolinium-based contrast medium that is excreted into the breast milk and absorbed
by the infant’s gut, the available data suggest that it is safe for the mother and infant to continue breast-feeding after receiving
such an agent [5].
Ultimately, an informed decision to temporarily stop breast-feeding should be left up to the mother after these facts are
communicated as survey data suggests that patient preference and radiologist’s opinion may sometimes differ [10]. If the mother
remains concerned about any potential ill effects to the infant, she may abstain from breast-feeding from the time of contrast
administration for a period of 12 to 24 hours. In this situation, the mother should be told to express and discard breast milk from
both breasts after contrast administration until breast feeding resumes. In anticipation of this, she may wish to use a breast pump
to obtain milk before the contrast-enhanced study to feed the infant during the 24-hour period following the examination. There
is little value to stop breast feeding beyond 24 hours, by which time the contrast media is nearly completely cleared from the
mother’s bloodstream.
References
1. Webb JA, Thomsen HS, Morcos SK. The use of iodinated and gadolinium contrast media during pregnancy and lactation. Eur Radiol
2005;15:1234-40.
2. Tremblay E, Thérasse E, Thomassin-Naggara I, Trop I. Quality initiatives: guidelines for use of medical imaging during pregnancy and lactation.
Radiographics 2012;32:897-911.
3. Wang PI, Chong ST, Kielar AZ, et al. Imaging of pregnant and lactating patients: part 1, evidence-based review and recommendations. AJR Am
J Roentgenol 2012;198:778-84.
4. Nielsen ST, Matheson I, Rasmussen JN, Skinnemoen K, Andrew E, Hafsahl G. Excretion of iohexol and metrizoate in human breast milk. Acta
Radiol 1987;28:523-6.
5. Kubik-Huch RA, Gottstein-Aalame NM, Frenzel T, et al. Gadopentetate dimeglumine excretion into human breast milk during lactation.
Radiology 2000;216:555-8.
6. Rofsky NM, Weinreb JC, Litt AW. Quantitative analysis of gadopentetate dimeglumine excreted in breast milk. J Magn Reson Imaging
1993;3:131-2.
7. Lin SP, Brown JJ. MR contrast agents: physical and pharmacologic basics. J Magn Reson Imaging 2007;25:884-99.
8. Themelin C, Pierron C, Calafat JF, de Beaufort C. Transient neonatal hypothyroidism secondary to postnatal maternal exposure to contrast
medium. BMJ Case Rep 2019;12.
9. Bettmann MA. Frequently asked questions: iodinated contrast agents. Radiographics 2004;24 Suppl 1:S3-10.
10. Lemmenmeier S, Boehm IB. Injection of iodinated contrast media in lactating women: Shall we continue or stop breastfeeding? Eur J Radiol
2024;175:111464.

TABLE 1: CATEGORIES OF ACUTE REACTIONS 95
The following describes a classification system for acute adverse reactions to iodinated and gadolinium- containing contrast media.
Acute adverse reactions can be either allergic-like or physiologic. Allergic-like reactions have clinical manifestations similar to
allergic reactions. They are termed “allergic-like” rather than just “allergic” because they are often idiosyncratic and may differ
immunologically from true allergies despite their similar clinical presentations. A history of prior allergic-like reaction may be an
indication for corticosteroid premedication prior to future contrast-enhanced studies that utilize a similar contrast material.
Physiologic reactions are not allergic-like and represent a physiologic response to the contrast material. A history of a prior
physiologic reaction is not an indication for corticosteroid premedication.
Assessment of reaction severity is somewhat subjective, and it is difficult to succinctly describe all possible degrees of reaction
severity. Sound clinical judgment should be used to determine when and how aggressively an acute reaction should be treated.
However, many mild reactions resolve during a period of observation without treatment.
Acute contrast reaction management and delayed allergic-like and non-allergic (e.g., CIN, NSF) adverse events to contrast media,
are described elsewhere in this Manual.
Mild
Signs and symptoms are self-limited without evidence of progression. Mild reactions include:
Allergic-like
Physiologic
Limited urticaria / pruritis
Limited nausea / vomiting limited
Cutaneous Edema
Transient flushing / warmth / chills
Limited “itchy”/“scratchy” throat
Headache / dizziness / anxiety / altered taste
Nasal congestion
Mild hypertension
Sneezing / conjunctivitis / rhinorrhea
Vasovagal reaction that resolves spontaneously
Moderate
Signs and symptoms are more pronounced and commonly require medical management. Some of these reactions have the
potential to become severe if not treated. Moderate reactions include:
Allergic-like
Physiologic
Diffuse urticaria / pruritis
Protracted nausea / vomiting
Diffuse erythema, stable vital signs
Hypertensive urgency
Facial edema without dyspnea
Isolated chest pain
Throat tightness or hoarseness without dyspnea
Vasovagal reaction that requires and is responsive to treatment
Wheezing / bronchospasm, mild or no hypoxia
Severe
Signs and symptoms are often life-threatening and can result in permanent morbidity or death if not managed appropriately.
Cardiopulmonary arrest is a nonspecific end-stage result that can be caused by a variety of the following severe reactions, both
allergic-like and physiologic. If it is unclear what etiology caused the cardiopulmonary arrest, it may be judicious to assume that
the reaction is/was an allergic-like one.
Pulmonary edema is a rare severe reaction that can occur in patients with tenuous cardiac reserve (cardiogenic pulmonary
edema) or in patients with normal cardiac function (noncardiogenic pulmonary edema). Noncardiogenic pulmonary edema can
be allergic-like or physiologic; if the etiology is unclear, it may be judicious to assume that the reaction is/was an allergic-like
one.
Table 1: CATEGORIES OF ACUTE REACTIONS
TABLE 1: CATEGORIES OF ACUTE REACTIONS 96
Severe reactions include:
Allergic-like
Physiologic
Diffuse edema, or facial edema with dyspnea
Vasovagal reaction resistant to treatment
Diffuse erythema with hypotension
Arrhythmia
Laryngeal edema with stridor and/or hypoxia
Convulsions, seizures
Wheezing / bronchospasm, significant hypoxia
Hypertensive emergency
Anaphylactic shock (hypotension + tachycardia)
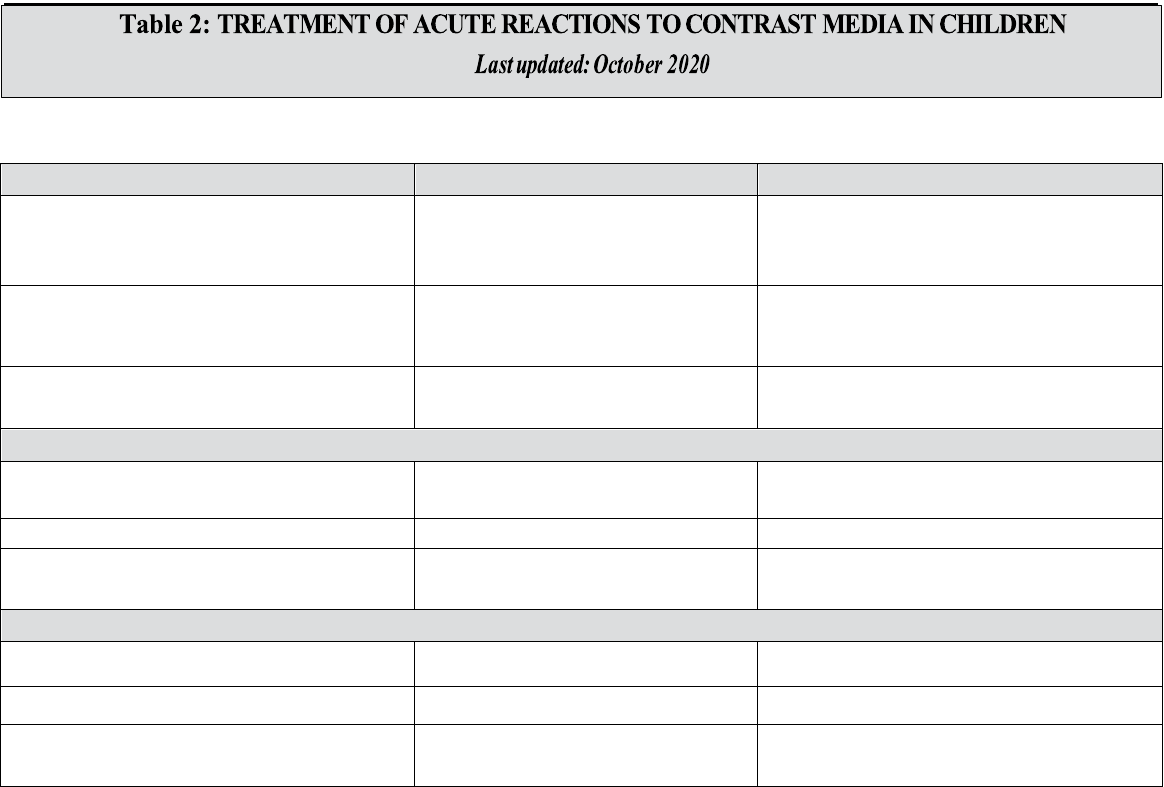
TABLE 2: TREATMENT OF ACUTE REACTIONS TO CONTRAST MEDIA IN CHILDREN 97
HIVES (Urticaria)
Treatment
Dosing
General comment: observe until hives are
resolving. Further observation may be
necessary if treatment is administered.
Mild (scattered and/or transient)
No treatment often needed; however, if
symptomatic, can consider
Diphenhydramine (Benadryl®)*
1 mg/kg (max = 50 mg) PO, IM, or IV;
administer IV dose slowly over 1 – 2 min
Moderate (more numerous/bothersome)
Monitor vitals
Preserve IV access
Consider
Diphenhydramine (Benadryl®)*
1 mg/kg (max = 50 mg) PO, IM, or IV;
administer IV dose slowly over 1 – 2 min
Severe (widespread and/or progressive)
Monitor vitals
Preserve IV access
Consider
Diphenhydramine (Benadryl®)*
1 mg/kg (max = 50 mg) PO, IM, IV;
administer IV dose slowly over 1 – 2 min
*Note: All forms can cause drowsiness; IV/IM form may cause or worsen hypotension.
Note: It can be difficult to dose medications accurately in neonates and infants. Also, with respect to IM delivery of epinephrine, EpiPen Jr®
package insert does not provide dosing recommendations for children < 15 kg.
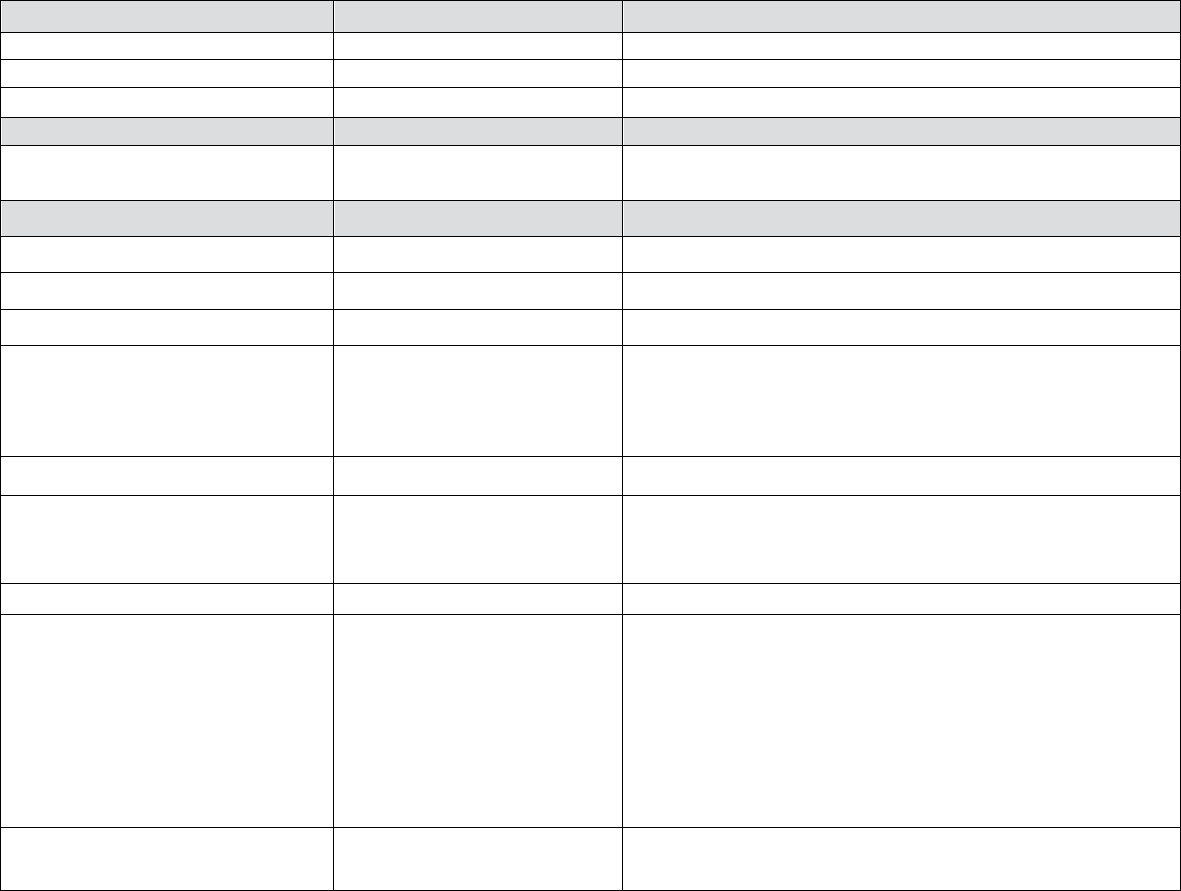
TABLE 2: TREATMENT OF ACUTE REACTIONS TO CONTRAST MEDIA IN CHILDREN 98
DIFFUSE ERYTHEMA
Treatment
Dosing
All forms
Preserve IV access
Monitor vitals
O2 by mask
6 – 10 L / min
Normotensive
No other treatment usually
needed
Treatment
Dosing
Hypotensive
IV fluids: 0.9% normal saline
10 – 20 mL / kg;
or
Maximum of 500 – 1,000 mL
Lactated Ringer’s
If profound or unresponsive to
fluids alone can also consider
Epinephrine (IV)*
IV 0.1 mL/kg of 0.1 mg/mL (1:10,000) dilution (0.01 mg/kg);
administer slowly into a running IV infusion of fluids; can repeat
every 5 – 15 min, as needed; maximum single dose: 1.0 mL (0.1
mg); can repeat up to 1 mg total dose
or (if no IV access available)
Epinephrine (IM)*
IM 0.01 mL/kg of 1.0 mg/mL (1:1,000) dilution (0.01 mg / kg);
max 0.30 mL (0.30 mg); can repeat every 5-15 minutes up to 1 mL
(1 mg) total
or
Epinephrine auto-injector
(1.0 mg/mL (1:1,000) dilution equivalent)
If < 30 kg, pediatric epinephrine auto-injector
(EpiPen Jr® or equivalent) 0.15 mL equivalent (0.15 mg);
If ≥ 30 kg, adult epinephrine auto-injector
(EpiPen® or equivalent) 0.30 mL (0.30 mg)
Consider calling emergency
response team or 911
*Note: In hypotensive patients, the preferred route of epinephrine delivery is IV, as the extremities may n ot be perfused sufficiently to allow for adequate
absorption of IM administration. Also, with respect to IM delivery of epinephrine, the EpiPen Jr® package insert does not provide dosing
recommendations for children < 15 kg.
Note: It can be difficult to dose medications accurately in neonates and infants.
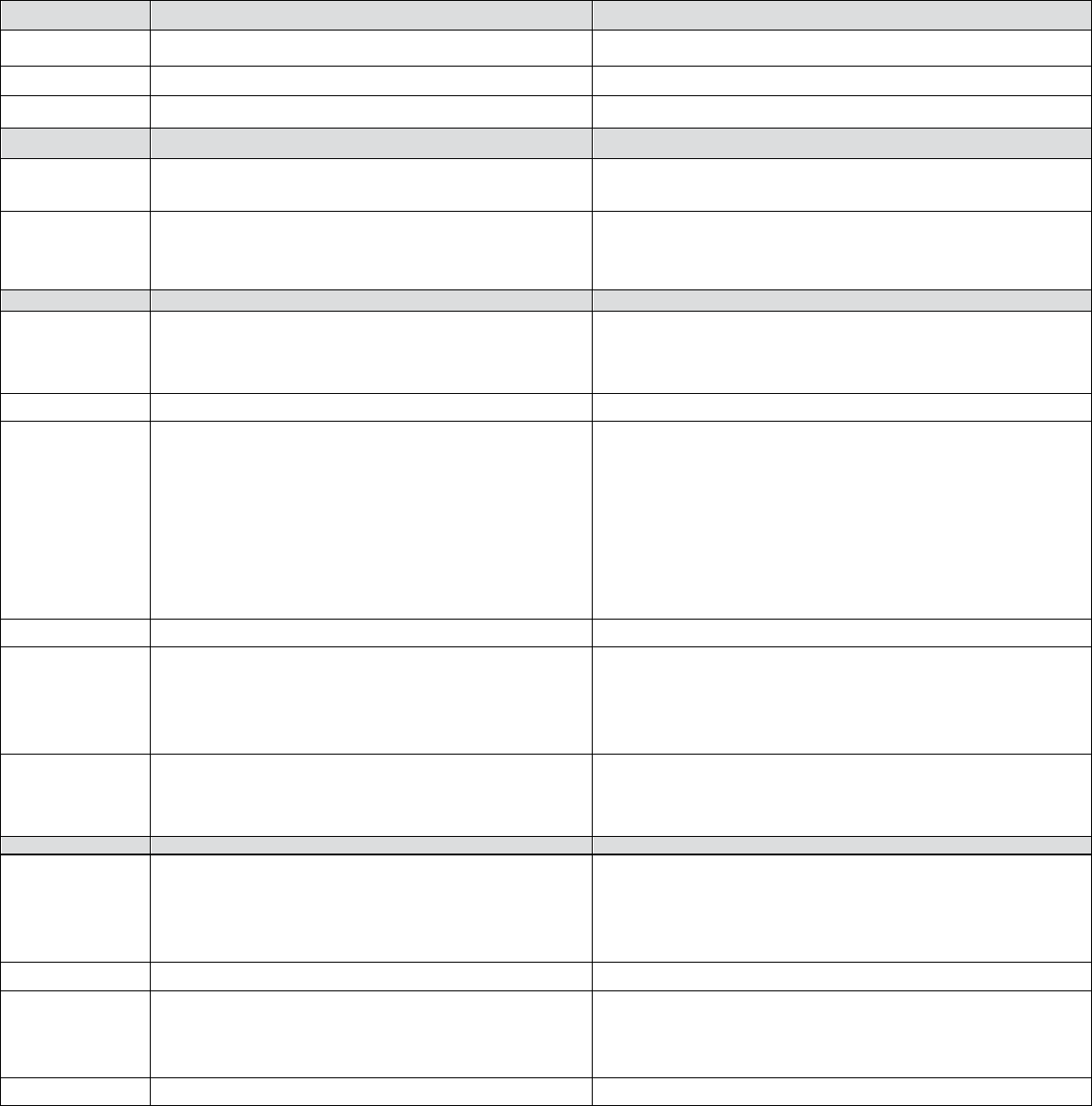
TABLE 2: TREATMENT OF ACUTE REACTIONS TO CONTRAST MEDIA IN CHILDREN 99
BRONCHOSPASM
Treatment
Dosing
All forms
Preserve IV access
Monitor vitals
O2 by mask
6–10 L / min
Treatment
Dosing
Mild
Beta agonist inhaler (Albuterol®)
2 puffs (90 mcg/puff) for a total of 180 mcg; can repeat up to 3
times
Consider calling emergency response team or 911,
based upon the completeness of the response
Moderate
Consider adding epinephrine (IM)*
IM 0.01 mL/kg of 1.0 mg/mL (1:1,000) dilution
(0.01
mg/kg); max 0.30 mL (0.30 mg); can repeat every 5-15
minutes up to 1 mL (1 mg) total
or
Epinephrine auto-injector
(1.0
mg/mL (1:1,000) dilution equivalent)
If < 30 kg, pediatric epinephrine auto-injector
(EpiPen Jr® or equivalent) 0.15 mL equivalent (0.15 mg);
If ≥ 30 kg, adult epinephrine auto-injector
(EpiPen® or equivalent) 0.30 mL (0.30 mg)
or
Epinephrine (IV)*
IV 0.1 mL/kg of 0.1 mg/mL (1:10,000) dilution (0.01 mg/kg);
administer slowly into a running IV infusion of fluids; can
repeat every 5–15 min, as needed; maximum single dose:1.0
mL (0.1 mg); can repeat up to 1 mg total dose
Consider calling emergency response team or 911 based
upon the completeness of the response
Severe
Epinephrine (IV)*
IV 0.1 mL / kg of 0.1 mg/mL (1:10,000) dilution (0.01 mg / kg);
administer slowly into a running IV infusion of fluids; can
repeat every 5-15 min, as needed; maximum single dose: 1.0 mL
(0.1 mg); can repeat up to 1 mg total dose
or
Epinephrine (IM)*
IM 0.01 mL/kg of 1.0 mg/mL (1:1,000) dilution (0.01 mg/kg);
max 0.30 mL (0.30 mg); can repeat every 5-15 minutes up to1
mL (1 mg) total
or

TABLE 2: TREATMENT OF ACUTE REACTIONS TO CONTRAST MEDIA IN CHILDREN 100
Treatment
Dosing
Epinephrine auto-injector
(1.0 mg/mL (1:1,000) dilution equivalent)
If < 30 kg, pediatric epinephrine auto-injector
(EpiPen Jr® or equivalent) 0.15 mL equivalent (0.15 mg);
If ≥ 30 kg, adult epinephrine auto-injector
(EpiPen® or equivalent) 0.30 mL (0.30 mg)
AND
Beta agonist inhaler (Albuterol®) (May work
synergistically)
2 puffs (90 mcg/puff) for a total of 180 mcg; can repeat up to 3
times
Call emergency response team or 911
*Note: In hypotensive patients, the preferred route of epinephrine delivery is IV, as the extremities may n ot be perfused sufficiently to allow for
adequate absorption of IM administration. Also, with respect to IM delivery of epinephrine, the EpiPen Jr® package insert does not provide dosing
recommendations for children < 15 kg.
Note: It can be difficult to dose medications accurately in neonates and infants.
LARYNGEAL EDEMA
Treatment
Dosing
All forms
Preserve IV access
Monitor vitals
O2 by mask
6–10 L/min
IV 0.1 mL/kg of 0.1 mg/mL (1:10,000) dilution (0.01 mg/kg);
administer slowly into a running IV infusion of fluids; can repeat
every 5–15 min, as needed; maximum single dose: 1.0 mL (0.1
mg); can repeat up to 1 mg total dose
or
Epinephrine (IM)*
IM 0.01 mL/kg of 1.0 mg/mL (1:1,000) dilution (0.01 mg/kg);
max 0.30 mL (0.30 mg); can repeat every 5-15 minutes up to 1
mL (1 mg) total
or
Epinephrine auto-injector (1:1,000 dilution equivalent)
If < 30 kg, pediatric epinephrine auto-injector
(EpiPen Jr® or equivalent) 0.15 mL equivalent (0.15 mg);
If ≥ 30 kg, adult epinephrine auto-injector
(EpiPen® or equivalent) 0.30 mL (0.30 mg)
Call emergency response team or 911
*Note: In hypotensive patients, the preferred route of epinephrine delivery is IV, as the extremities may n ot be perfused sufficiently to allow for
adequate absorption of IM administration. Also, with respect to IM delivery of epinephrine, the EpiPen Jr® package insert does not provide dosing
recommendations for children < 15 kg.
Note: It can be difficult to dose medications accurately in neonates and infants.

TABLE 2: TREATMENT OF ACUTE REACTIONS TO CONTRAST MEDIA IN CHILDREN 101
HYPOTENSION (minimum normal blood pressure varies for children of different ages)
Treatment
Dosing
All forms
Preserve IV access
Monitor vitals
O2 by mask
6–10 L/min
Elevate legs at least 60 degrees
Consider IV fluids: 0.9% normal saline
10–20 mL/kg;
or
Maximum of 500–1,000 mL
Lactated Ringer’s
Hypotension with bradycardia (min normal pulse varies for children of different ages) (Vasovagal reaction)
If mild
No other treatment usually necessary
If severe (patient remains
symptomatic despite above
measures)
In addition to above measures:
Atropine (IV)
IV 0.2 mL/kg of 0.1 mg/mL solution (0.02 mg/kg); Minimum
single dose = 0.1 mg
Maximum single dose = 0.6–1.0 mg Maximum total dose = 1 mg
for infants and children
2 mg for adolescents administer into a running IV infusion of
fluids
Hypotension with tachycardia (max normal pulse varies for children of different ages) (Anaphylactoid reaction)
If severe (hypotension persists)
Epinephrine (IV)*
IV 0.1 mL/kg of 0.1 mg/mL (1:10,000) dilution (0.01 mg/kg);
administer slowly into a running IV infusion of fluids; can repeat
every 5–15 min, as needed; maximum single dose: 1.0 mL (0.1
mg); can repeat up to 1 mg total dose
or
Epinephrine (IM)*
IM 0.01 mg/kg of 1.0 mg/mL (1:1,000) dilution (0.01 mL/kg);
max 0.30 mL (0.30 mg); can repeat every 5–15 minutes up to 1
mL (1 mg) total
or
Treatment
Dosing
Epinephrine auto-injector
(1.0
mg/mL (1:1,000) dilution equivalent)
If < 30 kg, pediatric epinephrine auto-injector
(EpiPen Jr® or equivalent) 0.15 mL equivalent (0.15 Mg);
If ≥ 30 kg, adult epinephrine auto-injector
(EpiPen® or equivalent) 0.30 mL (0.30 mg)
Call emergency response team or 911
*Note: In hypotensive patients, the preferred route of epinephrine delivery is IV, as the extremities may not be perfused sufficiently to allow for
adequate absorption of IM administration. Also, with respect to IM delivery of epinephrine, the EpiPen Jr® package insert doe s not provide
dosing recommendations for children < 15 kg.
Note: It can be difficult to dose medications accurately in neonates and infants.
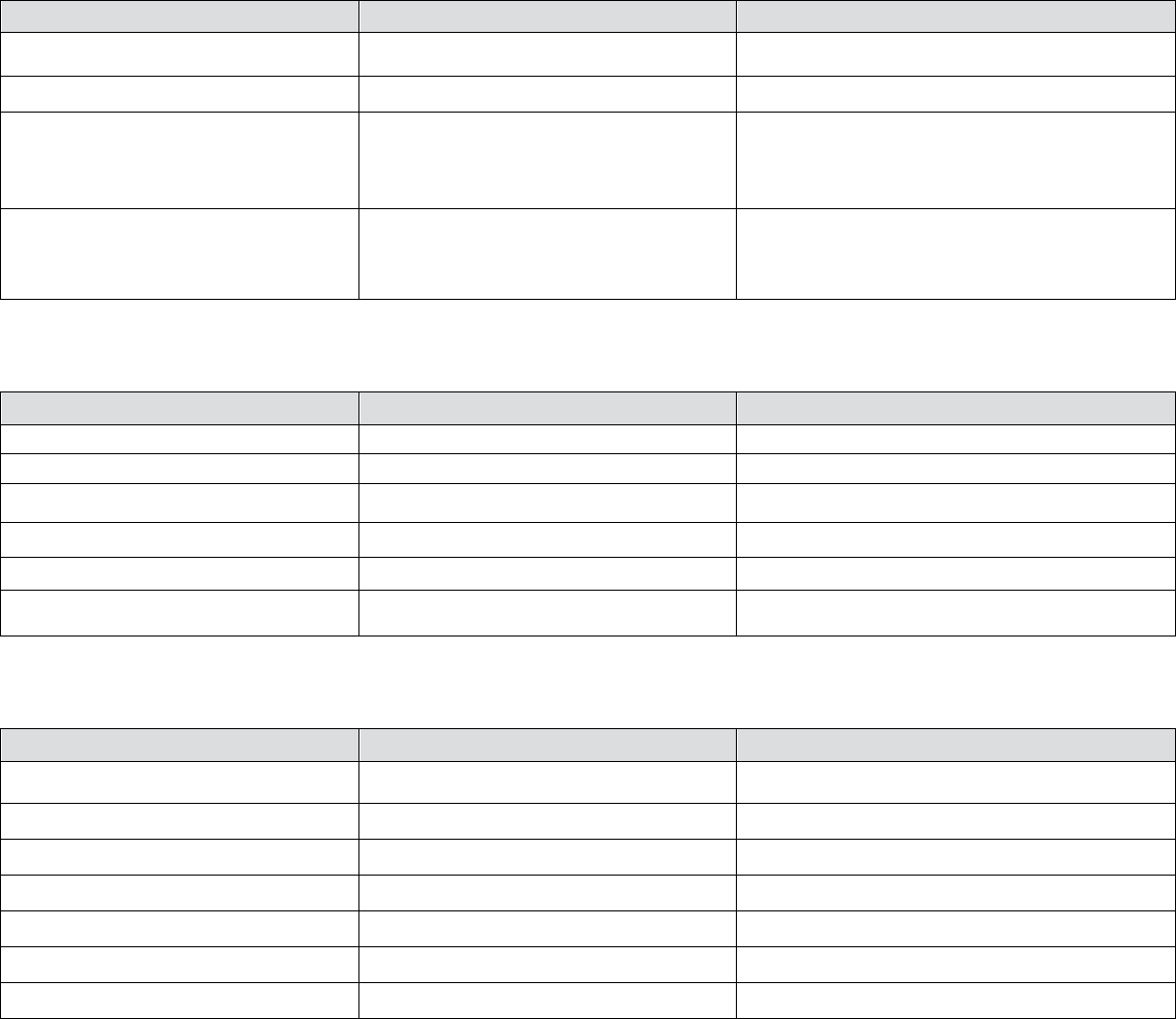
TABLE 2: TREATMENT OF ACUTE REACTIONS TO CONTRAST MEDIA IN CHILDREN 102
UNRESPONSIVE AND PULSELESS
Treatment
Dosing
Activate emergency response team (call 911)
Start CPR
Get defibrillator or automated electronic
defibrillator (AED); apply as soon as
available; shock as indicated
Note: Please also see BLS and
ACLS (PALS) booklets published by
the American Heart Association
Epinephrine (between 2 min cycles)
0.1 mL/kg of 0.1 mg/mL (1:10,000) dilution (0.01
mg/kg); administer quickly with flush or IV fluids;
max dose of 10 mL (1 mg)
PULMONARY EDEMA
Treatment
Dosing
Preserve IV access
Monitor vitals
O2 by mask
6–10 L/min
Elevate head of bed
Furosemide (Lasix®) (IV)
IV 0.5–1.0 mg/kg; over 2 min; maximum = 40 mg
Call emergency response team or 911
SEIZURES/CONVULSIONS
Treatment
Dosing
Observe and protect the patient
Turn patient on side to avoid aspiration
Suction airway, as needed
Preserve IV access
Monitor vitals
O2 by mask
6–10 L/min
If unremitting
Call emergency response team or 911
TABLE 2: TREATMENT OF ACUTE REACTIONS TO CONTRAST MEDIA IN CHILDREN 103
HYPOGLYCEMIA
Treatment
Dosing
All forms
Preserve IV access
O2 by mask
6–10 L / min
If patient is able to swallow safely
Observe
Administer oral glucose
2 sugar packets or 15 g of glucose tablet or gel or ½ cup (4 oz)
of fruit juice
If patient is unable to swallow safely
And IV access is available
Dextrose 50% (IV)
IV D25 2 mL/ kg; IV injection over 2 min
And IV access is not available
Glucagon (IM/SQ)
IM/SQ 0.5 mg if < 20 kg
IM/SQ 1.0 mg if > 20 kg
ANXIETY (PANIC ATTACK)
Treatment
Dosing
Diagnosis of exclusion
Assess patient for developing signs and symptoms that might indicate another type of reaction
Preserve IV access
Monitor vitals
Pulse oximeter
If no identifiable manifestations and normal oxygenation, consider this diagnosis
Reassure patient
REACTION REBOUND PREVENTION
Treatment
Dosing
Note: While IV corticosteroids may help prevent a short-term
recurrence of an allergic-like reaction, they are not useful in
the acute treatment of any reaction. However, these may be
considered for patients having severe allergic-like
manifestations prior to transportation to an Emergency
Department of inpatient unit.
Hydrocortisone
(Solu-Cortef®) (IV)
IV 5 mg/kg; administer over 1-2 min;
maximum: 200 mg
or
Methylprednisolone
(Solu-Medrol®) (IV)
IV 1 mg/kg; administer over 1–2 min;
maximum: 40 mg
Revision History
14 October 2020: Minor revisions
28 August 2015: Major revisions
15 May 2015: Major revisions
7 June 2013: Major revisions
17 March 2010: Minor revisions
29 October 2008: First version
TABLE 3: MANAGEMENT OF ACUTE REACTIONS TO CONTRAST MEDIA IN ADULTS 104
HIVES (Urticaria)
Treatment
Dosing
Mild (scattered and/or transient)
No treatment often needed; however, if
symptomatic, can consider:
Diphenhydramine (Benadryl®)*
25–50 mg PO
or
Fexofenadine (Allegra®)**
180 mg PO
Moderate (more numerous/bothersome)
Monitor vitals
Preserve IV access
Consider diphenhydramine (Benadryl®)*
25–50 mg PO
or
Fexofenadine (Allegra®}**
180 mg PO
or
Consider diphenhydramine (Benadryl®)*
25–50 mg IM or IV (administer IV dose
slowly over 1–2 min)
Severe (widespread and/or progressive)
Monitor vitals
Preserve IV access
Consider
Diphenhydramine (Benadryl®)*
25–50 mg IM or IV (administer
IV dose slowly over 1–2 min)
* Note: all forms can cause drowsiness; IM/IV
form may cause or worsen hypotension
** Note: second generation antihistamines cause
less drowsiness; may be beneficial for patients
who need to drive themselves home
Table 3: MANAGEMENT OF ACUTE REACTIONS TO CONTRAST MEDIA IN ADULTS
Last updated:
October
2020
TABLE 3: MANAGEMENT OF ACUTE REACTIONS TO CONTRAST MEDIA IN ADULTS 105
DIFFUSE ERYTHEMA
Treatment
Dosing
All forms
Preserve IV access
Monitor vitals
Pulse oximeter
O2 by mask
6–10 L/min
Normotensive
No other treatment usually needed
Treatment
Dosing
Hypotensive
IV fluids 0.9% normal saline
1,000 mL rapidly
or
Lactated Ringer’s
1,000 mL rapidly
If profound or unresponsive to fluids alone can
also consider
Epinephrine (IV)*
IV 1 mL of 0.1 mg/mL (1:10,000) dilution (0.1 mg);
administer slowly into a running IV infusion of fluids;
can repeat every few minutes as needed up to 10 mL (1
mg) total
or
(if no IV access available)
Epinephrine (IM)*
IM 0.3 mL of 1.0 mg/mL (1:1,000) dilution (0.3 mg);
can repeat every 5-15 minutes up to 1 mL (1 mg) total
or
Epinephrine auto-injector (EpiPen® or equivalent)
(0.3mL of 1.0 mg/mL (1:1,000) dilution, fixed [0.3mg]);
can repeat every 5-15 minutes up to three times
Consider calling emergency
response team or 911
* Note: in hypotensive patients, the preferred route
of epinephrine delivery is IV, as the extremities
may not be perfused sufficiently to allow for
adequate absorption of IM administered drug.
TABLE 3: MANAGEMENT OF ACUTE REACTIONS TO CONTRAST MEDIA IN ADULTS 106
BRONCHOSPASM
Treatment
Dosing
All forms
Preserve IV access
Monitor vitals
Pulse oximeter
O2 by mask
6–10 L/min
Mild
Beta agonist inhaler (Albuterol®)
2 puffs (90 mcg/puff) for a total of 180 mcg; can repeat up to 3
times
Consider sending patient to the Emergency Department or
calling emergency response team or 911, based upon the
completeness of the response to the beta agonist inhaler
Moderate
Beta agonist inhaler (Albuterol®)
2 puffs (90 mcg/puff) for a total of 180 mcg; can repeat up to 3
times
Consider adding epinephrine (IM)*
IM 0.3 mL of 1.0 mg/mL (1:1,000) dilution (0.3 mg); can repeat
every 5-15 minutes up to 1 mL (1 mg) total
or
Epinephrine auto-injector (EpiPen® or equivalent) (0.3 mL of 1.0
mg/mL (1:1,000) dilution, fixed [0.3mg]); can repeat every 5-15
minutes up to three times
or
Epinephrine (IV)*
IV 1 mL of 0.1 mg/mL(1:10,000) dilution (0.1 mg); administer
slowly into a running IV infusion of fluids or use saline flush; can
repeat every few minutes as needed up to 10 mL (1 mg) total
Consider calling emergency response team or 911 based
upon the completeness of the response
Severe
Epinephrine (IV)*
IV 1 mL of 0.1 mg/mL (1:10,000) dilution (0.1 mg); administer
slowly into a running IV infusion of fluids or slow IV push
followed by a slow saline flush; can repeat every few minutes as
needed up to 10 mL (1 mg) total
or
Epinephrine (IM)*
IM 0.3 mL of 1:1,000 dilution (0.3 mg); can repeat every 5-15
minutes up to 1 mL (1 mg) total
or
Epinephrine auto-injector (EpiPen® or equivalent) (0.3 mL of 1.0
mg/mL(1:1,000) dilution, Fixed [0.3mg]); can repeat every 5-15
minutes up to three times
AND
Beta agonist inhaler (Albuterol®) (may work
synergistically)
2 puffs (90 mcg/puff) for a total of 180 mcg; can repeat up to 3
times
Call emergency response team or 911
* Note: in hypotensive patients, the
preferred route of epinephrine delivery
is IV, as the extremities may not be
perfused sufficiently to allow for
adequate absorption of IM administered
drug.
TABLE 3: MANAGEMENT OF ACUTE REACTIONS TO CONTRAST MEDIA IN ADULTS 107
LARYNGEAL EDEMA
Treatment
Dosing
All forms
Preserve IV access
Monitor vitals
Pulse oximeter
O2 by mask
6–10 L/min
Epinephrine (IV)*
IV 1 mL of 0.1 mg/mL (1:10,000) dilution (0.1 mg);
administer slowly into a running IV infusion of fluids or
use saline flush; can repeat every few minutes as needed
up to 10 mL (1 mg) total
or
Epinephrine (IM)
IM 0.3 mL of 1.0 mg/mL (1:1,000) dilution (0.3 mg); can
repeat every 5-15 minutes up to 1 mL (1 mg) total
or
Epinephrine auto-injector (EpiPen® or equivalent) (0.3
mL of 1.0 mg/mL (1:1,000) dilution, fixed [0.3mg]); can
repeat every 5-15 minutes up to three times
Consider calling emergency
response team or 911 based upon
the severity of the reaction and the
completeness of the response
* Note: in hypotensive patients, the preferred
route of epinephrine delivery is IV, as the
extremities may not be perfused sufficiently to
allow for adequate absorption of IM
administered drug.
TABLE 3: MANAGEMENT OF ACUTE REACTIONS TO CONTRAST MEDIA IN ADULTS 108
HYPOTENSION
(systolic blood pressure< 90 mm Hg)
Treatment
Dosing
All forms
Preserve IV access
Monitor vitals
Pulse oximeter
Treatment
Dosing
O2 by mask
6–10 L/min
Elevate legs at least 60 degrees
IV fluids 0.9% normal saline
1,000 mL rapidly
or
Lactated Ringer’s
1,000 mL rapidly
Treatment
Dosing
Hypotension with bradycardia (pulse < 60 bpm) (Vasovagal reaction)
If mild
No other treatment usually necessary
If severe
(patient remains symptomatic despite above measures)
In addition to above measures: Atropine (IV)
0.6–1.0 mg; administer into a running IV infusion of
fluids; can repeat up to 3 mg total
Consider calling the emergency response team
or 911
Hypotension with tachycardia (pulse > 100 bpm) (Anaphylactoid reaction)
If hypotension persists
Epinephrine (IV)*
IV 1 mL of 0.1 mg/mL (1:10,000) dilution (0.1 mg);
administer slowly into a running IV infusion of
fluids; can repeat every few minutes as needed up to
10 mL (1 mg) total
or
Epinephrine (IM)*
IM 0.3 mL of 1.0 mg/mL (1:1,000) dilution (0.3 mg);
can repeat every 5-15 minutes up to 1 mL (1 mg)
total
or
Epinephrine auto-injector (EpiPen® or equivalent)
(0.3 mL of 1.0 mg/mL (1:1,000) dilution, fixed
[0.3mg]); can repeat every 5-15 minutes up to three
times
Consider calling emergency response team or
911 based upon the severity of the reaction and
the completeness of the response
* Note: in hypotensive patients, the preferred route
of epinephrine delivery is IV, as the extremities
may not be perfused sufficiently to allow for
adequate absorption of IM administered drug.
TABLE 3: MANAGEMENT OF ACUTE REACTIONS TO CONTRAST MEDIA IN ADULTS 109
HYPERTENSIVE CRISIS
(diastolic BP> 120mmHg; systolic blood pressure > 200mm Hg; symptoms of endorgan compromise)
Treatment
Dosing
All forms
Preserve IV access
Monitor vitals
Pulse oximeter
O2 by mask
6–10 L/min
Labetalol (IV)
20 mg IV; administer slowly, over 2 min; can double the
dose every 10 min (e.g., 40 mg 10 min later, then 80 mg 10
min after that)
or
(if labetalol not available)
Nitroglycerin tablet (SL)
0.4 mg tablet; can repeat every 5–10 min
and
Furosemide (Lasix®) (IV)
20–40 mg IV; administer slowly over 2 min
Call emergency response team or 911
PULMONARY EDEMA
Treatment
Dosing
Preserve IV access
Monitor vitals
O2 by mask
6–10 L/min
Pulse oximeter
Elevate head of bed, if possible
Furosemide (Lasix®)
20–40 mg IV; administer slowly over 2 min
Call emergency response team or 911
TABLE 3: MANAGEMENT OF ACUTE REACTIONS TO CONTRAST MEDIA IN ADULTS 110
SEIZURES/CONVULSIONS
Treatment
Dosing
Observe and protect the patient
Turn patient on side to avoid aspiration
Suction airway, as needed
Preserve IV access
Monitor vitals
Pulse oximeter
O2 by mask
6–10 L/min
If unremitting
Call emergency response team or 911
Treatment
Dosing
Lorazepam (IV)
IV 2–4 mg IV; administer slowly to maximum dose of 4 mg
HYPOGLYCEMIA
Treatment
Dosing
Preserve IV access
O2 by mask
6–10 L/min
If patient is able to swallow safely
Oral glucose
Two sugar packets or 15 g of glucose tablet/gel or ½
cup (4 oz) of fruit juice
If patient is unable to swallow safely and IV
access available
Dextrose 50% (IV)
D50W 1 ampule (25 grams) IV administer over 2
min
D5W or D5NS (IV) as adjunct therapy
Administer at a rate of 100 mL/hour
If no IV access is available
Glucagon (IM)
IM 1 mg
ANXIETY (PANIC ATTACK)
Treatment
Dosing
Diagnosis of exclusion
Assess patient for developing signs and symptoms that might indicate another type of reaction
Preserve IV access
Monitor vitals
Pulse oximeter
If no identifiable manifestations and normal oxygenation, consider this diagnosis
Reassure patient
TABLE 3: MANAGEMENT OF ACUTE REACTIONS TO CONTRAST MEDIA IN ADULTS 111
REACTION REBOUND PREVENTION
Treatment
Dosing
Note: While IV corticosteroids may help prevent a short-term
recurrence of an allergic-like reaction, they are not useful in the
acute treatment of any reaction. However, these may be
considered for patients having severe allergic-like manifestations
prior to transportation to an Emergency Department or inpatient
unit.
Hydrocortisone
(Solu-Cortef®) (IV)
IV 5 mg / kg; administer over 1-2 min
or
Methylprednisolone
(Solu-Medrol®) (IV)
IV 1 mg / kg; administer over 1-2 min
Revision History
14 October 2020: Minor revisions
28 August 2015: Major revisions
15 April 2013: Major revisions
26 June 2012: Minor revisions
23 June 2010: Major revisions
15 March 2004: First version

TABLE 4: EQUIPMENT FOR CONTRAST REACTIONS KITS IN RADIOLOGY 112
Facilities should be equipped with basic emergency equipment and medications needed to assess patients and treat contrast
reactions. Equipment that can help assess a patient’s clinical status include a stethoscope, blood pressure and pulse monitor,
and a pulse oximeter. While no standard contrast reaction kit exists, sites should consider making key medications available
for prompt reaction management. This would include epinephrine 1 mg/1 mL for intramuscular injection (this includes
standard Epinephrine auto-injectors), albuterol, and an antihistamine. Additional medications and equipment are listed in
Table 4. Due to financial and operational constraints related to frequent replacement of medications with a relatively short
shelf life, many practices are choosing to stock only essential medications separate from standard code carts. A periodic
monitoring program to ensure equipment functionality and medication shelf life is recommended.
Depending on the size and function of an imaging site, it may be sufficient to have one treatment cart designed for both
contrast reactions and cardiopulmonary arrest. Other facilities may find it more cost-effective to have separate contrast
reaction kits and code carts. Smaller distributed contrast reaction kits focused on the most frequently used or urgently needed
items can enable rapid implementation of treatment at considerably lower expense. In general, “code carts” designed for
treatment of cardiopulmonary arrest have more equipment than necessary for radiologists to use, and facilities may find the
suggestions below helpful in designing a dedicated reaction treatment cart that can be used to manage patients experiencing a
contrast reaction.
The contact phone number of the local emergency response team (if one is available) should be clearly posted within or near
any room in which contrast media is to be injected. If there is no emergency response team, the emergency external phone
number to be used (e.g., 911) should be displayed instead.
The following equipment is suggested to be readily available and within or nearby any room in which contrast media is to be
injected. Adult or pediatric sizes are optional for facilities that do not inject adult or pediatric patients, respectively. Sites may
opt to stock less equipment and medications if emergency response teams or ambulance support is readily available.
The following minimum equipment should be within or near any room in which contrast media is to be injected:
•
Access to oxygen
*
•
Defibrillator or automated external defibrillator (AED)
•
Blood pressure and pulse monitor
•
Pulse oximeter
•
Stethoscope
* Although oxygen can be administered in a variety of ways, use of non -rebreather masks is preferred because of their ability to deliver a larger dose
of oxygen to the patient.
The following minimum medications should be within or near any room in which contrast media is to be injected:
•
Epinephrine IM 1mg/1mL (auto-injector or vials with needle and syringe for use)
•
Inhaled short-acting beta-agonist (inhaler or nebulizer)
•
Anti-histamine
Table4:
EQUIPMENT FOR CONTRAST REACTION KITS IN RADIOLOGY
Last updated: January 2020
TABLE 4: EQUIPMENT FOR CONTRAST REACTIONS KITS IN RADIOLOGY 113
The following discretionary medications and equipment may be considered for inclusion within or near any room in which
contrast media is to be injected:
•
Equipment
o
Suction: wall-mounted or portable; tubing and catheters
o
“Ambu®-type” bag-valve-mask device; masks in adult and pediatric sizes; protective barriers for mouth-to-
mouth respiration optional if bag-valve-mask device is stocked
o
Normal saline (0.9%) and tubing
o
Syringes and IV cannulas: variety of sizes; tourniquets
o
Needle(s) for IM drug administration
•
Medications
o
Epinephrine IV 1mg/10mL, 10-mL preloaded syringe
o
Atropine IV, 1mg/10mL, 10-mL preloaded syringe
o
Corticosteroid IV
o
Nitroglycerin sublingual, 0.4 mg tab
o
Aspirin per oral, 325 mg (for chest pain where myocardial ischemia is a consideration)
o
Lasix IV, 20–40 mg (for pulmonary edema)
o
Labetalol IV, 20 mg (for hypertensive emergency)
o
Dextrose IV, 50% 25g/50mL syringe (for hypoglycemia)
APPENDIX A: CONTRAST MEDIA SPECIFICATION 114
INTRAVASCULAR
Product
Generic name (concentration in mg
contrast/ml)
Ionicity
Iodine+
(mg/ml)
Viscosity+
25°C
(cp or mPa.s)
Viscosity+37°C
(cp or mPa.s)
Osmolality
(mOsm/kgH2O)
Omnipaque™ 140 (GE Healthcare)
Iohexol 302
Nonionic
140
2.3*
1.5
322
Conray™ 30 (Covidien)
iothalamate (300)
Ionic
141
2
1.5
600
Ultravist® 150 (Bayer HealthCare)
iopromide
Nonionic
150
2.3*
1.5
328
Omnipaque™ 180 (GE Healthcare)
iohexol (388)
Nonionic
180
3.1*
2
408
Isovue®-200 (Bracco)
iopamidol (408)
Nonionic
200
3.3*
2.0
413
Conray™ 43 (Covidien)
iothalamate (430)
Ionic
202
3
2
1000
Omnipaque™ 240 (GE Healthcare)
iohexol (518)
Nonionic
240
5.8*
3.4
520
Optiray™ 240 (Guerbet)
ioversol (509)
Nonionic
240
4.6
3.0
502
Ultravist® 240 (Bayer Healthcare)
iopromide
Nonionic
240
4.9*
2.8
483
Isovue® 250 (Bracco)
iopamidol (510)
Nonionic
250
5.1*
3.0
524
Visipaque™ 270 (GE Healthcare)
iodixanol (550)
Nonionic
270
12.7*
6.3
290
Conray™ (Covidien)
iothalamate (600)
Ionic
282
6
4
1400
Isovue® 300 (Bracco)
iopamidol (612)
Nonionic
300
8.8*
4.7
616
Omnipaque™300 (GE Health care)
iohexol (647)
Nonionic
300
11.8*
6.3
672
Optiray™ 300 (Guerbet)
ioversol (640)
Nonionic
300
8.2
5.5
651
Oxilan® 300 (Guerbet)
ioxilan (623)
Nonionic
300
9.4*
5.1
610
Ultravist® 300 (Bayer Healthcare)
iopromide
Nonionic
300
9.2*
4.9
607
Hexabrix™*** (Guerbet)
Ioxaglate meglumine/sodium (589)
Ionic
320
15.7*
7.5
≈600
Optiray™320 (Guerbet)
ioversol (680)
Nonionic
320
9.9
5.8
702
Visipaque™ 320 (GE Healthcare)
iodixanol (652)
Nonionic
320
26.6
11.8
290
Optiray™ 350 (Guerbet)
ioversol (740)
Nonionic
350
14.3
9.0
792
Omnipaque™ 350 (GE Healthcare)
iohexol (755)
Nonionic
350
20.4*
10.4
844
Oxilan® 350 (Guerbet)
ioxilan (727)
Nonionic
350
16.3*
8.1
721
Isovue® 370 (Bracco)
iopamidol (755)
Nonionic
370
20.9*
9.4
796
MD-76™ R (Guerbet)
diatrizoate/ meglumine/sodium (760)
Ionic
370
16.4
10.5
1551
Ultravist® 370 (Bayer Healthcare)
Ioprokoi98mide
Nonionic
370
22.0*
10.0
774
Appendix A – CONTRAST MEDIA SPECIFICATIONS
Last updated: March 2023

APPENDIX A: CONTRAST MEDIA SPECIFICATION 115
+ Data from product package inserts, product brochures, technical information services and Rohrer, M, et al., Comparison of M agnetic Properties of MRI Contrast Media
Solutions at Different Field Strengths. Investigative Radiology 2005;40:715-724.
* Measured at 20℃.
** Data on file with Covidien
*** Hexabrix is a registered trademark of Guerbet, S.A. and is co-marketed in the U.S. by Guerbet LLC and Covidien.
o Viscosities of most products intended for oral administration are not reported by manufacturers.
# Barium concentrations are expressed as percent by weight (%w/w) and percent weight-in volume (% w/v). Percent by weight is the number of grams of barium sulfate per 100
grams of final suspension. For barium powders, percent by weight is the proportion of total powder weight that is pure barium, and the remainder is additives (Ex., barium
100% w/w is pure barium with no additives). Percent weight-in volume is the number of grams of barium sulfate per 100 mL of final suspension
1 Adopted from Reiter et al. Minimizing risk of nephrogenic systemic fibrosis in cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2012; 14(1): 31.
Cond 7.4 refers to value at physiologic pH of 7.4.
Appendix A –
CONTRAST MEDIA SPECIFICATIONS (continued)
GASTROINTESTINAL
– Non-Barium Oral Contrast
Product
Generic name
(concentration in mg
contrast/ml)
Ionicity
Iodine+
(mg/ml)
Viscosity+ 25°C
(cp or mPa.s)
Viscosity+
37°C
(cp or mPa.s)
Osmolality
(mOsm/kg
H2O)
Gastrografin® (Bracco)
diatrizoate meglumine
sodium (660)
Ionic
367
MD-Gastroview™ (Guerbet)
diatrizoate meglumine
sodium (660)
Ionic
367
Omnipaque™ 180 (GE Healthcare)
iohexol (388) pediatric use
Nonionic
180
3.1*
2.0
331
Omnipaque™ 240 (GE Healthcare)
iohexol (518) pediatric use
Nonionic
240
5.8*
3.4
520
Omnipaque™ 300 (GE Healthcare)
iohexol (647) pediatric use
Nonionic
300
11.8*
6.3
672
Omnipaque™ 350 (GE Healthcare)
iohexol (755) adult use
Nonionic
350
20.4*
10.4
844
Gastromark™ (Guerbet)
Discontinued in US
ferrous-ferric oxide
ferumoxsil
NA
NA
250
GASTROINTESTINAL
– Barium-Based Oral Contrast
Product
Chemical Structure
Concentration
(w/v or w/w)#
E-Z-HD (Bracco)
barium sulfate
98% w/w
Liquid Polibar Plus (Bracco)
barium sulfate
105% w/v or 58% w/w
Liquid Polibar (Bracco)
barium sulfate
105% w/v
E-Z-Paque / Pilibar ACB (Bracco)
barium Sulfate
96% w/w
Liquid E-Z-Paque (Bracco)
barium sulfate
60% w/v or 41% w/w
Readi-cat 2 (Bracco)
barium sulfate
2.1% w/v
E-Z-Paste (Bracco)
barium sulfate
60% w/w
Entero Vu (Bracco)
barium sulfate
24% w/v
Tagitol™ (Bracco)
barium sulfate
40% w/v or 30% w/w
Varibar® (Bracco)
Barium sulfate in variable consistency
40% w/v
E-Z-HD (Bracco)
barium sulfate
98% w/w
NeuLumEX
™
(E-Z-EM Inc/Bracco)
barium sulfate
0.1% w/v or 0.1% w/w

APPENDIX A: CONTRAST MEDIA SPECIFICATION 116
+ Data from product package inserts, product brochures, technical information services and Rohrer, M, et al., Comparison of Magnetic Properties of MRI
Contrast Media Solutions at Different Field Strengths. Investigative Radiology 2005;40:715-724.
* Measured at 20℃.
** Data on file with Covidien
*** Hexabrix is a registered trademark of Guerbet, S.A. and is co-marketed in the U.S. by Guerbet LLC and Covidien.
o Viscosities of most products intended for oral administration are not reported by manufacturers.
# Barium concentrations are expressed as percent by weight (%w/w) and percent weight-in volume (% w/v). Percent by weight is the number of grams of barium
sulfate per 100 grams of final suspension. For barium powders, percent by weight is the proportion of total powder weight that is pure barium and the remainder
is additives (Ex., barium 100% w/w is pure barium with no additives). Percent weight-in volume is the number of grams of barium sulfate per 100 mL of final
suspension
1 Adopted from Reiter et al. Minimizing risk of nephrogenic systemic fibrosis in cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2012; 14(1):
31. Cond 7.4 refers to value at physiologic pH of 7.4.
Appendix A
– CONTRAST MEDIA SPECIFICATIONS (continued)
GENITOURINARY
Product
Generic name (concentration in mg
contrast/ml)
Ionicity
Iodine+
(mg/ml)
Viscosity+
25°C
(cp or mPa.s)
Viscosity+
37°C
(cp or mPa.s)
Osmolality
(mOsm/kg
H2O
)
Cystografin® (Bracco)
diatrizoate
Ionic
141
Cysto-Conray™ II (Guerbet)
iothalamate (172)
(Only for retrograde cystography and
cystourethrography)
Ionic
81
(Only for retrograde cystography
and cystourethrography)
~400
Conray™ 43 (Guerbet)
iothalamate (430)
Ionic
202
3
2
1000
Omnipaque™ Can be diluted for
retrograde use. See package insert
iohexol
Nonionic
INTRATHECAL
Product
Generic name
(concentration in mg
contrast/ml)
Ionicity
Iodine+
(mg/ml)
Viscosity+
25°C
(cp or mPa.s)
Viscosity+
37°C
(cp or mPa.s)
Osmolality
(mOsm/kg H2O
)
Omnipaque™ 180 (GE Healthcare)
iohexol
Nonionic
180
3.1*
2.0
408
Omnipaque™ 240 (GE Healthcare)
iohexol
Nonionic
240
5.8*
3.4
520
Omnipaque™ 300 (GE Healthcare)
iohexol
Nonionic
300
11.8*
6.3
672
Isovue-M® 200 (Bracco)
iopamidol
Nonionic
200
3.3*
2.0
413
Isovue-M® 300 (Bracco)
iopamidol
Nonionic
300
8.8*
4.7
616
APPENDIX A: CONTRAST MEDIA SPECIFICATION 117
GADOLINIUM-BASED INTRAVASCULAR
Revision History
September 2020: Minor Revision
April 2023: Minor Revision
Product
Chemical Structure
and Class
Anion
Cation
Viscosity+
25°C
(cp or mPa.s)
Viscosity+
37°C
(cp or mPa.s)
Relaxivity
1.5T (3T)
Osmolality
(mOsm/kg H2O)
Log k Therm
(cond7.4)
Elucirem® (Guerbet)
Vueway® (Bracco)
Macrocyclic Non-ionic
Gadopiclenol****
None
12.6*
7.6
12.8 (11.6)
850
18.7
Magnevist®
(Bayer Healthcare)
Gd-DTPA
Linear Ionic
Gadopentetate
Dimeglumine
4.9*
2.9
4.1 (3.7)
1960
22.5 (18.4)
Prohance® (Bracco)
Gd-HP-D03A
Macrocyclic Non-ionic
Gadoteridol
None
2.0*
1.3
4.1 (3.7)
630
23.8 (17.2)
Multihance® (Bracco)
Gd-BOPTA
Linear Ionic
Gadobenate
Dimeglumine
9.2*
5.3
6.3 (5.5)
1970
22.6 (18.4)
Omniscan™ (GE Healthcare)
Gd-DTPA-BMA
Linear Non-ionic
Gadodiamide
None
2.0
1.4
4.3 (4)
789
16.9 (14.9)
Optimark™ (Guerbet)
Gd-DTPA-BMEA
Linear Non-ionic
Gadoversetamide
None
2.8**
2.0
4.7 (4.5)
1110
16.8 (15)
EOVIST/Primovist®
(Bayer Healthcare)
Gd-EOB-DTPA
Linear Ionic
Gadoxetate
Disodium
1.19
6.9 (6.2)
688
23.5 (18.7)
Gadavist/Gadovost™
(Bayer Healthcare)
Gd-BT-D03A
Macrocyclic Non Ionic
Gadobutrol
None
4.96
5.2 (5)
1603
21.8 (15.5)
Dotarem® (Guerbet)
Clariscan™ (GE Healthcare)
Gd-DOTA
Macrocyclic Ionic
Gadoterate
Meglumine
3.4*
2.4
3.6 (3.5)
1350
25.6 (19.3)
Data from product package inserts, product brochures, technical information services and Rohrer, M, et al., Comparison of Magnetic Properties of MRI Contrast Media Solutions
at Different Field Strengths. Investigative Radiology 2005; 40:715-724.
* Measured at 20℃.
** Data on file with Covidien
*** Hexabrix is a registered trademark of Guerbet, S.A. and is co-marketed in the U.S. by Guerbet LLC and Covidien.
o Viscosities of most products intended for oral administration are not reported by manufacturers.
# Barium concentrations are expressed as percent by weight (%w/w) and percent weight-in volume (% w/v). Percent by weight is the number of grams of barium sulfate per 100
grams of final suspension. For barium powders, percent by weight is the proportion of total powder weight that is pure barium, and the remainder is additives (Ex., barium 100%
w/w is pure barium with no additives). Percent weight-in volume is the number of grams of barium sulfate per 100 mL of final suspension
1 Adopted from Reiter et al. Minimizing risk of nephrogenic systemic fibrosis in cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2012; 14(1): 31. Cond 7.4 refers
to value at physiologic pH of 7.4
****Gadopiclenol demonstrates kinetic stability and a long dissociation half-life that are comparable to other Group II macrocyclic agents. Based on the most recent scientific and
clinical evidence, the ACR Committee on Drugs and Contrast Media considers the risk of NSF among patients exposed to standard or lower than standard doses of Gadopiclenol is
sufficiently low or possibly nonexistent such that it has been classified as a Group II agent.
